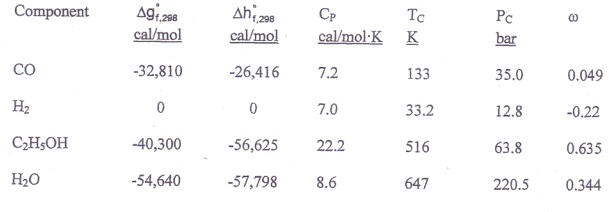
Ethanol is manufactured from carbon monoxide and hydrogen at 600 K and 20 bars according to the reaction
2 C0(g) + 4 H2(g) ↔ C2H5OH(g) + H2O (g)
The feed stream contains 60 mol% H2, 20 mol% CO and 20 mol% inerts.
a) Using only the data presented below, calculate the equilibrium constant Kf at 600 K.
b) Calculate the maximum conversion of carbon monoxide that can be attained in the reactor using
i) the ideal gas model, and
ii) the Lewis-Randall's approximation.
Data: