Assignment:
Q1. Write a structural formula for each of the following acids:
a. 4-oxohexanoic acid
b. 2-hydroxy-3-methylhexanoic acid
c. 2-chloropentanedioic acid
d. p-bromophenylacetic acid
Q2. Name each of the following acids.
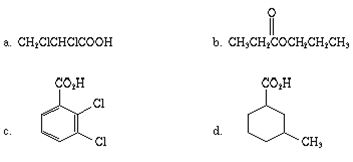
Q3. In each of the following pairs of acids, which would be expected to be stronger, and why?
a. CH2ClCH2CO2H or CH3CHClCO2H
b. m-ClC6H4CO2H or p-ClC6H4CO2H
Q4. Give equations for the synthesis of
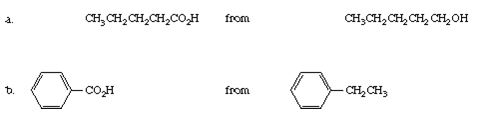
Q5. Write a structure for each of the following compounds:
a. trichloromethylformate
b. 2-chlorophenyl acetate
c. ethyl benzoate
d. sodium butanoate
Q6. Write an equation for the reaction of phenyl propanoate with
a. hot aqueous sodium hydroxide
b. ammonia (heat)
c. propylmagnesium bromide (two equivalents), then H3O+
d. lithium aluminum hydride (two equivalents), then H3O+
Q7. Write out all the steps in the mechanism for
a. saponification of ethyl benzoate
b. ammonolysis of ethyl benzoate
Q8. Write an equation for
a. esterification of propanoic acid with benzyl alcohol
b. oxidation of toluene with potassium permanganate
c. reduction of propylcyclopentane carboxylate with lithium aluminum hydride
Q9. Write an equation for saponification of glyceryltripalmitate.
Q10. Write an equation for hydrogenation of glyceryltrioleate.
Provide complete and step by step solution for the question and show calculations and use formulas.