Assignment:
Question 1. 5 mol of an ideal gas is kept at 140?C in an expansion from 1 L to 5 L .
How much work is done by the gas? The universal gas constant is 8.31 J/K/mol .
Question 2. Steam moves into the cylinder of a steam engine at a constant pressure and does 7.410 J of work on a piston. The diameter of the piston is 2.00 cm, and the piston travels 10.3 cm in one stroke.
What is the pressure of the steam?
Question 3. Gas in a container is at a pressure of 2.1 atm and a volume of 5 m3
What is the work done on the gas if it expands at constant pressure to three times its initial volume? 1.013 × 105 Pa = 1 atm.
Question 4. What is the work done on the gas if it is compressed at constant pressure to one-third of its initial volume?
Question 5. If the gas in a container absorbs 200 J of heat, has 119 J of work done on it, then does 30 J of work, what is the increase in the internal energy of the gas?
Question 6. One mole of gas is initially at a pressure of 2.1 atm, volume of 0.29 L and has an internal energy equal to 82.9 J. In its final state the gas is at pressure 0.9 atm and volume 0.92 L, and its internal energy equals 203 J.
.jpg)
For the path IAF, calculate the work done on the gas. 101300 Pa = 1 atm.
Question 7. For the path IBF, calculate the work done on the gas.
Question 8. For the path IF, calculate the work done on the gas.
Question 9. For the path IAF, find the net energy transferred to the gas by heat in the process.
Question 10. For the path IBF, find the net energy transferred to the gas by heat in the process.
Question 11. For the path IF, find the net energy transferred to the gas by heat in the process.
Question 12. An engine using 1 mol of an idea gas initially at 16 L and 322 K performs a cycle consisting of four steps:
1) an isothermal expansion at 322 K from 16 L to 37 L ,
2) cooling at constant volume to 163 K ,
3) an isothermal compression to its original volume of 16 L, and
4) heating at constant volume to its original temperature of 322 K .
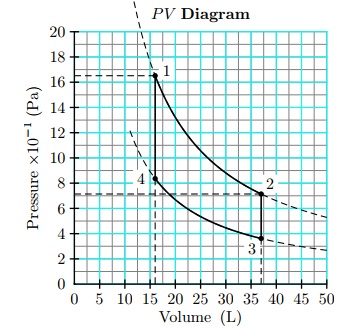
Assume that the heat capacity at constant volume is 21 J/K and the universal gas constant is 0.08206 L · atm/mol/K = 8.314 J/mol/K Verify the cycle on the P V diagram and find its efficiency.
Answer in units of percent.
Question 13. How much work is done by the steam when 1.2 mol of water at 125 ?C boils and becomes 1.2 mol of steam at 125?C at 1.2 atm pressure? The universal gas constant is 8.31451 J/K · mol .
Question 14. Find the change in internal energy of the steam as it vaporizes. Consider the steam to be an ideal gas with heat of aporization 2.26 × 106 J/kg.
Answer in units of kJ.
Question 15. A house has a surface temperature of 23?C. The surrounding temperature outside is 0?C. If the surface area of the house is 200 m2 and the emissivity is 1, find the energy radiated per second. Deal only with heat transfer due to infra-red electromagnetic radiation.
The Stefan-Boltzmann constant is 5.6696 × 10-8 W/m2· K 4
Question 16. A box with a total surface area of 1.36 m2 and a wall thickness of 5.53 cm is made of an insulating material. A 13.7 W electric heater inside the box maintains the inside temperature at 15.6 ?C above the outside temperature.
Find the thermal conductivity of the insulating material.
Answer in units of W/m ?C.
Question 17. The surface temperature of the Sun is about 5844 K.
Taking the radius of the Sun to be 6.94 × 108 m, calculate the total energy radiated by the Sun each second. Assume the emissivity
of the Sun is 0.965. The Stefan-Boltzmann constant is 5.6696 × 10-8 W/m2· K 4
Answer in units of W.
Question 18. Helium is used to fill a balloon of diameter 55 cm at 30?C and 2 atm.
The mass of a helium atom is 4.0026 u, the universal gas constant id 8.31451 J/K mol, Boltzmann's constant is 1.38066 × 10-23 J/K, and Avogadro's number is 6.022 × 1023 1/mol. 1.66 × 10-27 kg = 1 u, and 101300 Pa = 1 Pa.
How many helium atoms are there in the balloon?
Question 19. What is the average kinetic energy of each helium atom?
Question 20. What is the average speed (i.e., the root mean square speed) of each helium atom?
Answer in units of m/s.
Question 21. One mole of air at 320 K confined in a cylinder under a heavy piston occupies a volume of 5 L.
The heat capacity of air under constant volume is 52R.
Find the new volume of the gas if 5.5 kJ is transferred to the air.
Answer in units of L.
Question 22. Air in the cylinder of a diesel engine at 50?C is compressed from an initial pressure of 1.032 atm and of volume of 650 cm3 to a volume of 67.7 cm3. Assuming that air behaves as an ideal gas (γ = 1.40) and that the compression is adia batic and reversible, find the final pressure.
Answer in units of atm.
Question 23. Find the final temperature under the same assumptions as above.
Answer in units of ?C.