Assignment:
For this lab activity, we are going to be using the simulation available at https://phet.colorado.edu/sims/html/ph-scale/latest/ph-scale_en.html.
Part 1: pH of Common Solutions
- Before you begin the simulation, take a moment to complete the table below. Based on your knowledge from this week's lecture and your personal experience, predict what the pH of each solution would be:
|
Solution
|
Predicted pH
|
|
Drain cleaner
|
|
|
Hand soap
|
|
|
Blood
|
|
|
Spit
|
|
|
Milk
|
|
|
Chicken soup
|
|
|
Coffee
|
|
|
Orange juice
|
|
|
Soda pop
|
|
|
Vomit
|
|
|
Battery acid
|
|
- Access the lab and select the "Macro" option
- You will see the following screen:
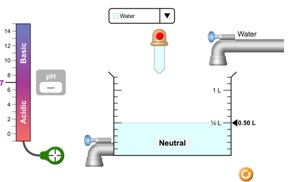
Notice there is no reading on the pH scale. That's because the "detector" (green circle, bottom left) is not in the tank. To measure the pH of your solution, you need to drag the detector to the tank.
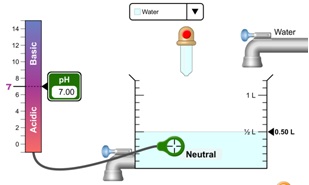
Notice that the scale now reads 7.0, which is the pH of pure water.
- Using the drop down menu at the top, one at a time, select the alternate solutions and record their actual pH in the table below:
|
Solution
|
Actual pH
|
|
Drain cleaner
|
|
|
Hand soap
|
|
|
Blood
|
|
|
Spit
|
|
|
Milk
|
|
|
Chicken soup
|
|
|
Coffee
|
|
|
Orange juice
|
|
|
Soda pop
|
|
|
Vomit
|
|
|
Battery acid
|
|
- Answer the following questions about your results:
o What facts did you use to predict the pH values of the solutions? How did your predicted pH values for each of the common solutions compare with the actual pH values for those solutions?
o Of the solutions you tested, which was the most acidic? Which was the most basic? Which was the closest to neutral?
o Milk of magnesia is sometimes used as a remedy for an "acid stomach". Would you expect the pH of milk of magnesia to be less than 7, more than 7, or 7? Why?
Part 2: Effects of Dilution and Mixing Solutions
- Answer this question before completing the next activity: What will happen to the pH of an acidic solution if you add water to dilute the solution? Why?
- Select orange juice from the drop down to fill the tank with 0.5L of OJ.
- Pull out the stopper on the water faucet and add 0.5L to the tank, for a total volume of 1L
- Measure the pH of the diluted OJ. Was your prediction correct? If not, can you explain why?
- Based on these results, if you added a basic solution to the acidic orange juice instead of plain water, what do you think would be the effect on the pH of the resulting mixture? Why?
Bonus: What are some real-world applications in which pH is an important factor? If you use an internet source to answer this question, please provide the link.