Question: The potential energy between two atoms can be represented as shown below:
V = - (A/r) + (B/r10)
Where A and B are constants and r is the inter atomic separation distance. Plot the potential energy verses distance relationships for these atoms when A = 8 x 10-30 J.m and B = 5 x 10-119 J.m10. Find out the coefficient of thermal expansion (in m/m.K) for the material over the given temperature ranges:
a) 0oK to 0oC,
b) 0oC to 820oC.
Suppose that thermal energy of the system is 3kT/2 where k is Boltzman’s constant k = 1.381 x 10-23 J/K.
Question: Gallium arsenide (GaAs) has the zinc blende structure. The gallium atoms are in FCC packing and the arsenic atoms occupy half the tetrahedral sites. Compute the density of Gallium arsenide (GaAs) by using the given data.
Covalent Radius Atomic Mass
Ga 1.26 Ångstroms 69.7 g/mole
As 1.19 Ångstroms 74.9 g/mole
Avogadro’s number (NAv) is 6.02 x 1023 atoms per mole.
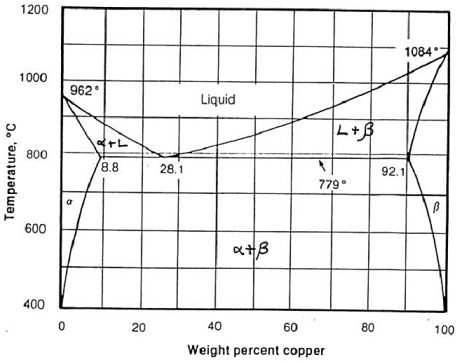
Question: Using the phase diagram below for the copper-silver system, find out:
i) The phases present
ii) The composition (wt % Cu) of those phases and
iii) The amount (wt %) of each phase for:
a) Copper-silver alloy (70 wt % copper) at 900 oC
b) Copper-silver alloy (70 wt % copper) at 600 oC
c) Copper-silver alloy (5 wt % copper) at 800 oC