Solve the below problem:
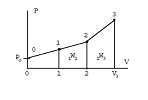
Q: Calculate the heat transfer for the process described. Two springs with same spring constant are installed in a mass less piston/cylinder with the outside air at 100 kPa. If the piston is at the bottom, both springs are relaxed and the second spring comes in contact with the piston at V = 2 m3. The cylinder contains ammonia initially at -2°C, x = 0.13, V = 1 m3, which is then heated until the pressure finally reaches 1200 kPa. At what pressure will the piston touch the second spring? Find the final temperature and the total work done by the ammonia.