Assignment:
“ Transfer the amide to a well-drained reaction flask, add 5 mL of concentrated hydrochloric acid and 10 mL of water (for each 5 g of aniline), and boil the mixture gently until the solid has all dissolved (5-10 min); then continue the heating at the boiling point for an additional 10 min (do not evaporate to dryness). The solution when cooled to room temperature should deposit no solid amide, but, if it is deposited, heating should be continued for a further period. The cooled solution of sulfanilamide hydrochloride is shaken with decolorizing charcoal and filtered by suction.
Place the solution in a beaker and cautiously add an aqueous solution of 5 g of sodium bicarbonate with stirring to neutralize the hydrochloride. After the foam has subsided, test the suspension with litmus; if it is still acidic, add more bicarbonate until the neutral point is reached. Cool thoroughly in ice and collect the granular, white precipitate of sulfanilamide
Clean up: add the water from the gas trap to the combined aqueous filtrates from all reactions and neutralize the solution by adding either 10% hydrochloric acid or sodium carbonate. Flush the neutral solution down the drain with a large excess of water. Any spilled drops of chlorosulfonic acid should be covered with sodium carbonate; then resulting powder should be collected in a beaker, dissolved in water and then flushed down the drain.”
Using the paragraph above, answer the following question.
1)After completion of the hydrolysis, the acidic reaction mixture will be neutralized with sodium bicarbonate and sulfanilamide precipitate. By reference to a chemical equation explain why neutralizing the aqueous acid causes sulfanilamide to precipitate. Illustrate your answer if necessary.
2) Show a step by step mechanism for the above reaction.
3)Show by calculation how much NaHCO3 is required to neutralize the HCl reaction mixture. Assume you have 5 g p-acetamidobenzenesulfonamide to hydrolyze. Concentrated HCl is 12 M.
4) What is the overall yield for a five-step synthesis with individual yields of 70%, 85%, 67%, 82%, and 90%?
5) Write acid-base chemical equations to explain the solubility of sulfanilamide in dilute NaOH and dilute HCl.
6) What product would be expected if 4-acetamidobenzenesulfonamide were subjected to vigorous hydrolysis conditions, such as concentrated hydrochloric acid and heat for a long period of time?
7) Sulfadiazine is a useful antimalarial drug. Suggest a reasonable synthesis of this drug starting from 2-aminopyrimidine and nitrobenzene. Show a step-by-step mechanism to make this product.
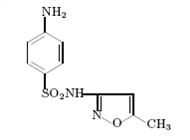
8)
Provide complete and step by step solution for the question and show calculations and use formulas.