Lab Assignment:
Title of Laboratory: Preparation and Use of Para Red Dye
Purpose: Diazonium salts are formed from the reaction of nitrous acid and a primary amine. You will prepare an azo dye from 2-napthol and p-nitroaniline. The extensively conjugate double bonds of the product results in an intensely colored product. The dye will be used to treat cotton fabric.
Goals:
• To prepare a diazonium salt
• To couple a diazonium salt with an activated aromatic ring system
• To collect, dry, weight and calculate percent yield of the para red dye.
• To treat white cotton fabric with the dye.
• To write the reactions representing the dye formation.
Materials:
• 2-Napthol
• p-Nitroaniline
• Sodium nitrate
• Concentrated H2SO4
• 2.5 M NaOH
• Glassware
• Vacuum Filtration System
• White cotton fabric
• Gloves
Procedure/Observations:
A. Preparation of Diazonium Salt
1. Mixed 0.5 mL concentrated H2SO4 in 10.0 mL water in a test tube.
2. Weighed out 0.22 g p-Nitroaniline and place it in the acid solution. Stir until you dissolve the p-nitroaniline. Continued to add H2SO4 dropwise until the solid is completely dissolved. Cooled it in an ice bath. Product was yellow in color.
3. Dissolved 0.13 g NaNo2 in 5 mL water. Added it to the p-nitroaniline solution. Mixed; recorded any observations. The solution became foggier and became a dark yellow color.
4. The diazonium salt was placed on ice until you are ready to use it.
B. Preparation of 2-Naphthol
1. Weighed out 0.25 g 2-naphtol and placed in a test tube.
2. Dissolved 5.0 mL 2.5 M NaOH. Stirred to help dissolve into solution.
3. Added additional 5.0 mL water to the test tube.
4. Placed in crushed ice until chilled. Product was a light brown.
C. Dying Fabric
1. Measured out approximately one-half of the diazonium salt into a 250 mL beaker. Added 50 mL cold water.
2. Placed fabric to be dyed in the beaker. Stirred it around and allowed it to sit for a few minutes.
3. Measured out approximately one-half of the alkaline 2-nathol solution and placed it in a clean 250 mL beaker. Added about 50 mL water.
4. Grabbed the fabric with forceps and press against the side of the beaker to expel as much liquid as possible, Placed the fabric in the 2-napthol solution and stirred it around. Allowed to sit for several minutes.
5. Removed the cloth sample and placed in beaker of water to rinse our excess dye. Set the fabric on a paper towel to dry.
D. Isolating the Para Red Dye.
1. Combined the remaining diazonium salt and 2-naphthol in a clean beaker. Stirred for a few minutes.
2. To the beaker added the contents of the two beakers from part C and stirred as before.
3. Acidified the mixture with 1 M H2SO4 so a precipitate is formed.
E. Filtration of product
1. Pre-weighed a watch glass and small filter paper, weighing 39.4952g.
2. Filtered the product using the vacuum filtration system and the filter paper that was weighed.
3. Washed the product with 5 mL deionixed water.
4. Using a spatula, removed the filter paper and dye to the watch glass.
5. Allowed to air dry before re-weighing. Total weight of watch glass, filter paper and dye was 39.6330, resulting in 0.1378g of dye.
F. Disposal
1. Placed the solid dye, original filtrate, and the dye solution in the appropriate waste containers.
Discussion:
Q1. Why is p-nitroaniline soluble in acid solution? Write the reaction.
Q2. Why is 2-naphthol soluble in base solution. Write the reaction.
Q3. Write the reactions for the preparation of the diaonuim salt for the coupling of the salt with the sodium salt of 2-napthol. See diagram below and narrative of the complete process.
Q4. Write the reaction of the sodium salt of para red when treated with 1 M H2SO4. Why does a precipitate form?
Q5. Determine the percent yield of your product para red dye. Base your calculation on the amount of 2-naphthol that was weighed out.
Q6. Why is the diazonium salt kept cold during formation and storage? Write the reaction for its decomposition to a phenol.
The following explanation covers the synthesis of Para Red from aniline.
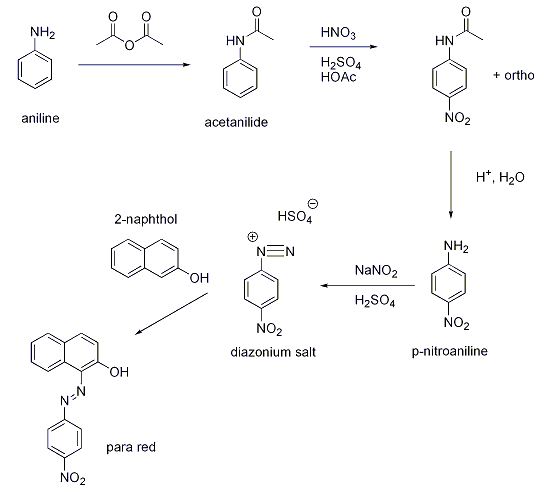
1. React aniline with acetic anhydride. This forms acetanilide. Collect the product by vacuum filtration.
2. Dissolve the acetanilide into glacial acetic acid. Add dropwise sulfuric and nitric acid. This forms p-nitroacetanilide. Cool, and collect precipitate.
3. Convert the p-nitroacetanilide into p-nitroaniline by reaction in water with acid catalyst. This hydrolyzes the amide bond in p-nitroacetanilide.
4. React the p-nitroaniline with sulfuric acid and sodium nitrite to form the diazonium salt. Keep cool, of course since diazonium salts decompose rapidly due to loss of N2 gas.
5. Prepare a cool solution of 2-naphthol in sodium hydroxide. Add the solution which has the diazonium salt in it to the basic 2-naphthol solution.
6. After the reaction has taken place, acidify the contents with H2SO4. The reason we treat this with sulfuric acid at this point is to protonate the phenoxide group on the naphthol ring portion. Remember, the 2-naphthol solution was prepared by dissolving it in sodium hydroxide. This would have formed the naphthoxide ion as the sodium hydroxide reacted with the phenol group on the 2-naphthol. By re-acidifying, we protonate this O- group forming the protonated phenol again. This makes the dye insoluble in acidic solution which forms the red precipitate, the Para Red dye.
References:
https://web.centre.edu/muzyka/organic/lab/paraRed05.htm.
Provide complete and step by step solution for the question and show calculations and use formulas.