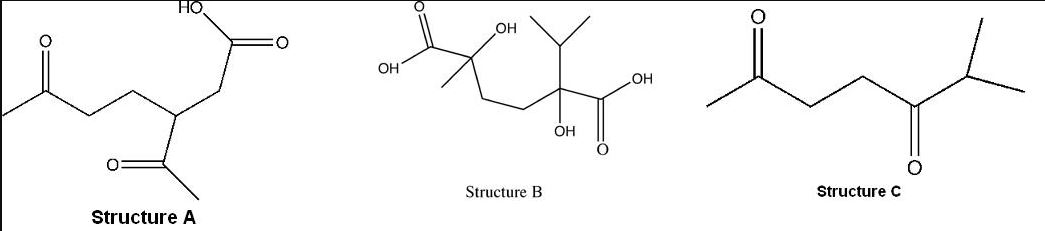
Assignment:
Limonene, C10H16, a terpene found in citrus peel, absorbs only two moles of hydrogen forming p-menthane C10H20. Oxidation by permanganate converts limonene into structure A (ignore structures B and C for this problem).
Q1. How many rings, if any, are in limonene?
Q2. What structures are consistent with the oxidation products?
Q3. On the basis of the isoprene rule, which structure is most likely for limonene? For p-menthane?
Q4. Addition of 1 mole H2O converts limonene into an alcohol. What are the most likely structures for this alcohol?
Q5. Addition of two moles of H2O to limonene yields terpin hydrate. What is the most likely structure for terpin hydrate?
Provide complete and step by step solution for the question and show calculations and use formulas.