Discuss the below:
1)You found the multiplicity for an ideal monatomic gas that lives in a two-dimensional universe. It was .
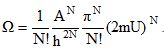
As implied, the multiplicity is determined by the internal energy, the area occupied by the gas, and the number of particles in the gas. From these, determine the temperature, ‘pressure', and chemical potential of this gas. (As a side note: in two dimensions, the ‘pressure' is defined as force per length).