Assignment:
Concept Explorations
Best Lewis Formula and Molecular Geometry
A student writes the Lewis electron-dot formula for the carbonate anion, CO32-, as
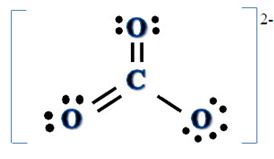
a. Does this Lewis formula obey the octet rule? Explain. What are the formal charges on the atoms? Try describing the bonding for this formula in valence bond terms. Do you have any difficulty doing this?
b. Does this Lewis formula give a reasonable description of the electron structure, or is there a better one? If there is a better Lewis formula, write it down and explain why it is better.
c. The same student writes the following resonance description for CO2: Is there something wrong with this description? (What would you predict as the geometries of these formulas?)
d. Is one or the other formula a better description? Could a value for the dipole moment help you decide?
e. Can you write a Lewis formula that gives an even better description of CO2? Explain your answer.
Molecular Geometry and Bonding
Draw four different Lewis electron-dot formulas for the SeO2 molecule. (HINT: One should have two single bonds, two have a single and a double bond, and one two double bonds).
a. Are there any electron-dot formulas that you expect to give the best description? How would you describe the bonding in terms of electron-dot formulas?
b. Describe the bonding in SeO2 in valence bond terms. If there is delocalized bonding, note this in your description and explain how molecular orbital theory can be useful here.
c. Determine the arrangement of electron pairs about Se in the SeO2 molecule. What would you expect for the molecular geometry of the SeO2 molecule?
d. Would you expect the O-Se-O angle in the SeO2 molecule to be greater than, equal to, or less than 120°. Explain your answer.
e. Draw an electron-dot formula for the H2Se molecule and determine its molecular geometry.
f. Compare the H-Se-H bond angle to the O-Se-O bond angle. Is it larger or smaller? Explain.
g. Determine whether the H2Se and SeO2 molecules have dipole moments. Describe how you arrived at your answer.
Provide complete and step by step solution for the question and show calculations and use formulas.