Assignment:
Q1. Write the complete stepwise mechanism for this reaction. Be sure to show all intermediate structures and all electron flow using arrows.
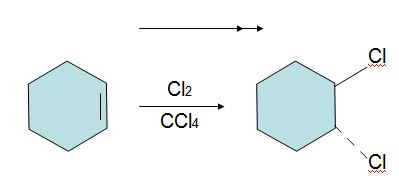
Q2. Predict the products of the following reaction. Indicate regiochemistry and stereochemistry when relevant.
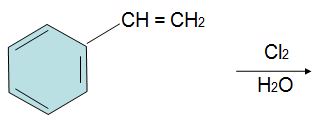
Q3. Provide a name for the compound below.
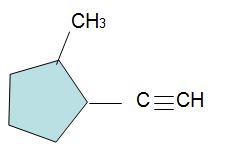
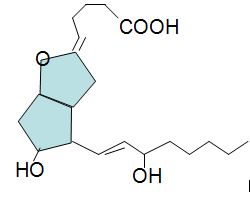
The compound below has been isolated from the safflower plant. Consider its structure to answer the following questions.
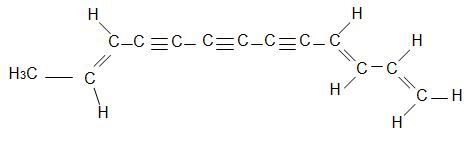
Q4. What is the molecular formula for this natural product?
Q5. What is the degree of unsaturation for this compound?
Q6. Assign E or Z configuration to each of the double bonds in the compound. Draw these above.
Q7. Provide the name for this unusual natural product.
Q8. Predict the product of the reaction below. Be sure to indicate stereochemistry when appropriate.
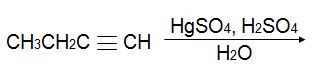
Q9. (2R,3S)-Dibromobutane is:
a. optically active
b. racemic
c. dextrorotory
d. a meso compound
Q10. Estriol, a potent estrogenic hormone, has been isolated from the urine of pregnant women. When 40 mg of estriol is dissolved in 1.0 mL of dioxane and placed in a sample tube with 1 dm path length a rotation of +2.32 is observed. Calculate the specific rotation for estriol.
Q11. (S)-()-Serine:
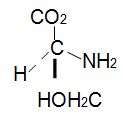
a. is dextrorotatory
b. rotates plane-polarized light in a counterclockwise direction
c. rotates plane-polarized light in a clockwise direction
d. is racemic
Q12. Draw the enantiomer of (S)-()-Serine in a wedge-dash projection.
Q13. Assign R, S configurations to each indicated chirality center in the molecule below. The configuration of this carbon atom is (red arrow)_______________.
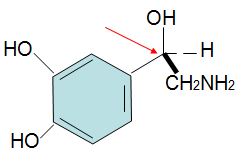
Q14. Assign R, S configurations to each indicated chirality center in the molecule below. The configuration of this carbon atom is (red arrow)_______________.
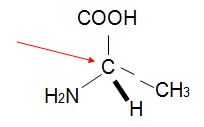
Q15. Assign R, S configurations to each indicated chirality center in the molecule below. The configuration of this carbon atom is (red arrow)_______________.
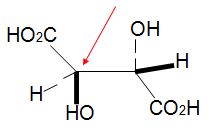
Q16. Assign R, S configurations to each indicated chirality center in the molecule below. The configuration of this carbon atom is (red arrow)_______________.
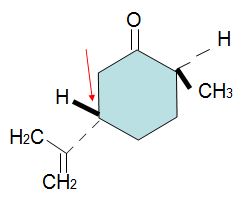
Select the best reagent or sequence of reagents from the list provided which would best accomplish each transformation below (numbers 17 & 18).
a. H2, Lindlar
b. 1. BH3, THF
2. H2O2
c. 1. NaNH2, NH3
2. CH3CH2I
d. 1. Br2, CCl4
2. 2 NaNH2, NH3
e. 1. HCl
2. NaNH2, NH3
f. Li/NH3
Q17.
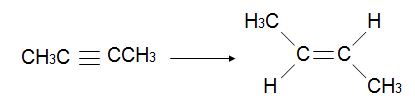
Q18.
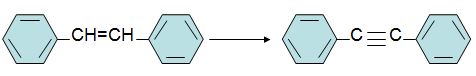
Q19. Place asterisks at all the chirality centers in the molecule below.
Provide complete and step by step solution for the question and show calculations and use formulas.