Assignment:
Question 1. Identify the conjugate acids of the bases C5H5N (pyridine), HPO42-, O2-, CH3COOH, [Co(CO)4]-, CN-.
Question 2. Use Pauling's rules to place the following acids in order of increasing acid strength: HNO2, H2SO4, HBrO3, and HClO4 in a nonlevelling solvent.
Question 3. Which member of the following pairs is the stronger acid? Give reasons for the choice.
[Fe(OH2)6]3+ or [Fe(OH2)6]2+
Question 4. Arrange the oxides Al2O3, B2O3, BaO, CO2, Cl2O7, SO3 in order from the most acidic through amphoteric to the most basic?
Question 5. Which of the following reactions are predicated to have an equilibrium constant greater than 1? Unless otherwise stated, assume gas-phase or hydrocarbon solution and 25oC.
R3PBBr3 + R3NBF3 à R3PRBF3 + R3NBBr3
Question 6. Predict whether the equilibrium constants for the following reactions should be greater than 1 or less than 1
[CuI4]2-(aq) + [CuCl4]3-(aq) à [CuCl4]2-(aq) + [CuI4]3-(aq)
Question 7. Balance the following redox reaction in acid solution: MnO4- + H2SO3 à Mn2+ + HSO4-. Predict the qualitative pH dependence on the net potential for this reaction (that is, increases, decreases, remains the same).
Question 8. Use standard potentials as a quide to write equations for the main net reaction that you would predict in the following experiments
I2 is added to excess aqueous HClO3.
Question 9. Characterize the condition of acidity or basicity that would most favour the following transformations in aqueous solution.
ClO4- à ClO3-
Question 10. Give an example of an acidic, a basic, and an amphoteric oxide from the same family in the Periodic Table.
Question 11. Identify the following as Lewis acids or bases and rank them in order of increasing hardness. Explain the reasoning.
O(CH3)2 S(CH3)2 Se(CH3)2
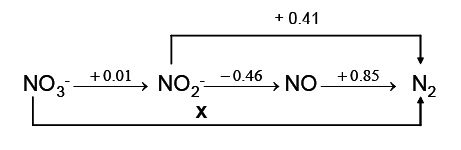
Question 12. The figure below is part of the modified Latimer diagram for nitrogen under conditions of 1.0 M OH-(aq). Identify the species in the Latimer diagram provided that are unstable with respect to disproportionation in 1.0 M hydroxide. State your reasoning. Calculate x.
Question 13. Write and balance the equation for the disproportionation of sulfite (SO32-) into sulfate and sulfide ions in basic solution.