Answer the following questions :
Part:1
1. A solution where water is the solvent is called a(n) solution.
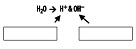
2. Label the diagram with the following terms: hydroxide ion and hydrogen ion.
3. What are two negative effects of too much acid in nature?
4. Complete the pH scale with the following terms/phrases: greater H+, lower H+, H+ = OH-.
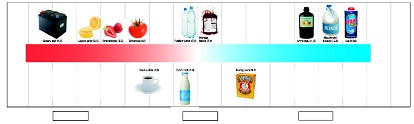
5. As the pH increases the [ H+ ] ____, and as the pH decreases the [ H+ ] ____.
A) decreases; increases
B) increases; decreases
C) increases; stays the same
D) decreases; stays the same
6. Complete the following table regarding acids and bases.
Acids Bases
Effect on H+ when dissolved in H2O
pH range
Example
7. The pH in your cells is dropping. You have buffers to minimize this change in pH. Briefly explain what the buffer would do in this situation with respect to the H+ concentration in your cells.
Part. 2:All life on Earth is based on carbon.
1. The element is essential to an organic compound.
2. What is meant by the term carbon skeleton, and how can carbon skeletons vary?
3. Is the following molecule an organic compound?
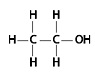
4. The oxygen atom in ethyl alcohol forms a polar covalent bond with the carbon atom. Does ethyl alcohol readily interact with water? Hint: Revisit module 2.5 if necessary.
5. Identify any functional groups from the following molecule.
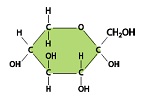
6. help to determine the overall properties of molecules.
7. Complete the table that describes the four classes of large biological molecules.
Carbohydrates Proteins Lipids Nucleic acids
Example
Function of example
Part. 3: Most biological macromolecules are polymers.
1. Much of your mass consists of large biological molecules called .
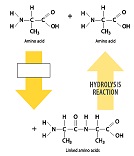
2. _________________ is the process by which polymers are broken into monomers.
A) Metabolism
B) Dehydration synthesis
C) Macromolecules
D) Hydrolysis
3. A common polymer is starch. We break starch down for use as an energy source. Is starch digestion hy-drolysis or dehydration synthesis?
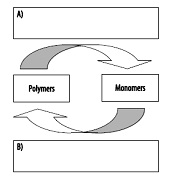
4. Complete the following diagram using the following terms: dehydration synthesis and hydrolysis.
5. Which of the following best describes the following process: glucose + glucose→ maltose + H2O?
A) Polarity
B) Hydrolysis
C) Metabolism
D) Dehydration synthesis
6. Complete the following diagram illustrating dehydration synthesis and hydrolysis.
7. is the total of all reactions that take place in your cells.
8. How are dehydration synthesis and hydrolysis opposites of each other?
Part. 4:Carbohydrates are composed of monosaccharides.
1. Which of the following is not a characteristic of carbohydrates?
A) Energy source for animals
B) Inclusion of monosaccharides
C) Use as a structure in plants
D) All of the above
2. The monomers of carbohydrates are .
3. Glucose is a monosaccharide. Is glucose also a carbohydrate? Briefly explain your answer.
4. Glucose and fructose both have the molecular formula C6H12O6, yet they are different molecules. Glu-cose and fructose are .
5. Two monosaccharides joined by a dehydration synthesis would form a(n) . List two exam-ples of a disaccharide.
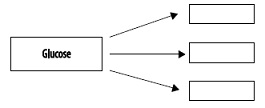
6. Complete the following illustration regarding monosaccharides and polysaccharides. Glucose is used to construct which polysaccharides?
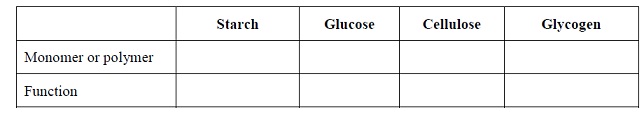
7. Complete the following table regarding monosaccharides and polysaccharides.
Part. 5:Lipids are a diverse group of hydrophobic molecules.
1. The one feature common to all lipids is that they are .
2. A tanker is carrying crude oil from Alaska to a port in California. During the trip, the ship's hull is rup-tured and it spills the crude oil into the Pacific Ocean. The oil sits on top of the water and does not mix with it. What characteristic must be true about the oil?
3. A cell's membrane consists, in large part, of a molecule called a phospholipid. Does a phospholipid "love" or "fear" water? Briefly explain your answer.
4. Complete the following diagram of a phospholipid.
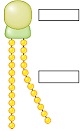
5. The two layers of phospholipids that make up a cell's membrane are arranged so that they are facing tail to tail. What would be the effect of this interior core of phospholipid tails? Hint: Keep in mind what you already know about phospholipid tails.
6. True or false: Cholesterol is used within the cell to help maintain the structure of the cell's membrane.
7. Which of the following is not a lipid?
A) Triglycerides
B) Cholesterol
C) Anabolic steroids
D) All of the above
8. Are both steroids and triglycerides in your body? If so, give a function performed by each.
Part. 6: Your diet contains several different kinds of fats.
1. There are two kinds of triglycerides. What are they?
2. Which fatty acid tail from the following diagram is saturated? Which one is unsaturated? Briefly ex-plain your answers.
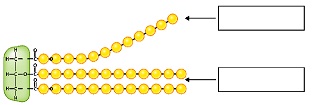
3. Saturated means that an object can hold no more of something. Briefly explain why the terms saturated and unsaturated are good descriptors for the molecules they describe.
4. You are a biochemist working for a food manufacturer. You are working to identify a new fat you have discovered in a plant from a rainforest of Brazil. During your investigation, you determine that the fat tends to be a liquid at room temperature, and a biochemical analysis reveals too few hydrogen atoms for the amount of carbon that is present. What kind of fat have you likely discovered?
5. An unsaturated fat can be turned into a solid or semi-solid state by __________________________.
A) omega-3 fatty acidification
B) hydrophilic
C) hydrogenation
D) unsaturation
6. Two students are discussing triglycerides. One student tells the other that all fats are bad for your health. The second student disagrees with that statement. Which student is correct? Briefly explain your an-swer.
Part. 7: Proteins perform many of life's functions.
1. A protein's determines its function.
2. _____ are the monomers from which large proteins are constructed.
A) Polymers
B) Amino acids
C) Polypeptides
D) Peptide bonds
3. Identify the peptide bond in the following illustration.
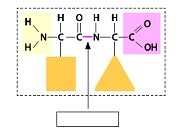
4. You are a biochemist working for a pharmaceutical company. You are identifying a new molecule you have isolated from a species of bat. You determine that the molecule contains a carboxylic acid group and a group of atoms that would give the molecule unique hydrophilic properties. The power goes out before you can finish your analysis. Based on what you know so far, what kind of molecule is it? What else might you have learned from it if the power had not gone out?
5. True or false: Amino acids are joined together through a dehydration synthesis reaction forming a pep-tide bond.
6. Complete the table on protein structure.
Polypeptide Folded chain Multiple chains
Description
7. Proteins serve many functions within your body. List three functions served by proteins in your body. Additionally, list a specific protein that performs that function.
8. Protein shape is crucial to its proper function. Students have difficulty grasping this idea and how changing shape affects function. Briefly explain how the words tasty and nasty can serve as a good anal-ogy for a teacher trying to explain this concept to students.
Part. 8: Enzymes speed chemical reactions.
1. A protein that speeds up chemical reactions is a(n) .
2. True or false: A chemical reaction changes the shape of the enzyme permanently. If false, make it a true statement.
3. Complete the following diagram regarding enzymes and substrates.
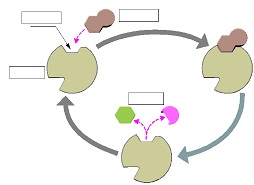
4. Briefly explain what would happen if you altered the shape of an enzyme's active site. Remember that an enzyme is a protein.
5. The amount of energy needed to perform a chemical reaction is the __________________________.
A) activation energy
B) substrate energy
C) active site
D) inhibition site
6. Complete the following diagram, which illustrates activation energy.
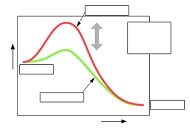
7. In pole vaulting, the higher the bar is placed, the more difficult it is to clear it. Explain why this is a good analogy to help students understand enzymes and activation energy.