Assignment:
An enzyme is a large protein molecule (typically with a molar mass greater than 20,000 AMU) that has a structure capable of catalyzing specific biological reactions. In general one or more reactant molecules (called substrates) bind to an enzyme at its "active sites" where the reaction the enzyme is responsible for occurs. The general mechanism for enzyme catalysis is:
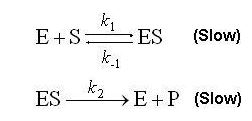
Where E stands for the enzyme, S the substrate, ES a short-lived intermidiate enzyme-substrate complex, and P the product of the reaction.
When solving this rate expression it is helpful to replace [E], the total enzyme concentration with [E]0 the initial amount of enzyme present which during the reaction is equal to:
[E]0 = [E] + [ES]
since it is very hard to measure the quantity of enzyme bound to the substrate [ES] during the reaction. This substitution will help you in solving this problem. Use the steady state approximation we learned in class to derive an expression for the rate of formation of product, d[P]/dt, that is only a function of [E]0 (defined above), [S], and the rate constants. Your expression should be independent of [ES] (the quantity of enzyme-substrate present), and [E] (the quantity of unbound enzyme), as these are very hard to measure during a reaction.
The equation you will be deriving was originally derived by Michaelis and Menten and is the standard equation used by biologists to describe most enzyme kinetics.
To help keep the input of your answer simple please make the following letter substitutions (note answers are CASE sensitive, use caps and lower case as defined here):
[E]0 = e k1 = a k2 = c
[S] = S k-1 = b
d[P]/dt =
Provide complete and step by step solution for the question and show calculations and use formulas.