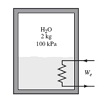
Solve the below problem:
Q: A well-insulated rigid tank contains 2 kg of a saturated liquid-vapor mixture of water at 100 kPa. Initially, three-quarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is now turned on and kept on until all the liquid in the tank is vaporized. Determine the entropy change of the steam during this process.