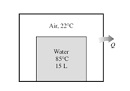
Solve the below problem:
Q: A 0.04-m3 tank initially contains air at ambient conditions of 100 kPa and 22°C. Now, a 15-liter tank containing liquid water at 85°C is placed into the tank without causing any air to escape. After some heat transfer from the water to the air and the surroundings, both the air and water are measured to be at 44°C. Determine
(a) The amount of heat lost to the surroundings and
(b) The energy destruction during this process.