Solve the following problem:
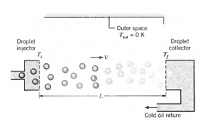
Q: As permanent space stations increase in size, there is an attendant increase in the amount of electrical power they dissipate. To keep station compartment temperatures from exceeding prescribed limits, it is necessary to transfer the dissipated heat to space. A novel heat rejection scheme that has been proposed for this purpose is termed a Liquid Droplet Radiator (LDR). The heat is first transferred to a high vacuum oil, which is then injected into outer space as a stream of small droplets. The stream is allowed to traverse a distance L, over which it cools by radiating energy to outer space at absolute zero temperature. The droplets are then collected and routed back to the space station.
Consider conditions for which droplets of emissivity e = 0.95 and diameter D = 0.5 mm are injected at a temperature of Tj = 500 K and a velocity of V = 0.1 m/s. Properties of the oil are p = 885 kg/m 3 , c = 1900 J/kg · K, and k = 0.145 W 1m . K. Assuming each drop to radiate to deep space at Tsur = 0 K, determine the distance L required for the droplets to impact the collector at a final temperature of Tf = 300 K. What is the amount of thermal energy rejected by each droplet?