Assignment:
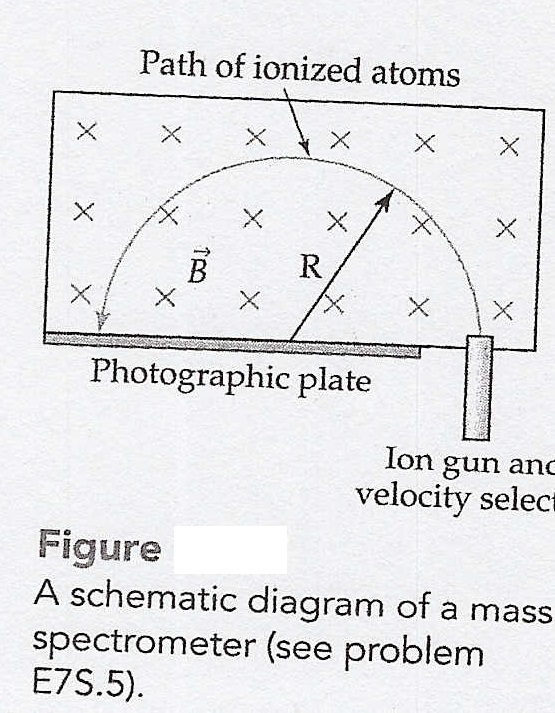
A mass spectrometer is a device that uses a magnetic field to sort atoms by mass. In this device, atoms whose masses are to be determined are ionized by stripping off one electron. They are then sent through a velocity selector that selects ions only with specific speed V, and these ions are sent into a region of space filled with a known uniform magnetic field B(vector) perpendicular to V(vector). The field causes each atom to follow a circular path whose radius is proportional to the atom's mass. Atoms with different masses will therefore follow somewhat different circular paths and thus end up at different places on a photographic plate, as shown in figure E7.17(included as attachment). Imagine that we give N2+ ions, O2+ ions, and NO+ ions the same velocity of 30.0km/s and then send them into a mass spectrometer where the magnetic field strength is B=8.5 MN/C. How far would the spot on the photographic plate be from the entry point for each ion, assuming that each ion completes one-half of an orbit, as shown in the figure? The atomic mass of a nitrogen atom is 14.0031 amu and oxygen is 15.9949 amu, where 1 amu= 1.6605 * 10^-27 kg.
Provide complete and step by step solution for the question and show calculations and use formulas.