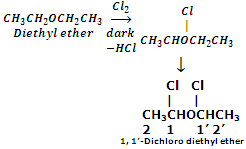
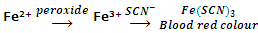Halogenations: ethers react with chlorine and bromine to give substitution products. The extent of halogenations depends upon the conditions of reactions.
In dark, diethyl ether produces 1, 1'-dichloro diethyl ether
In the presence of sunlight all the hydrogen's of ether are substituted by halogen atoms.
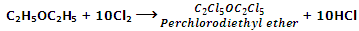
Peroxide formation: ethers in contact with air and light undergo oxidation to produce peroxides which hare unstable compounds and explode on heating.
Serious accidents are likely to occur if during distillation of old samples of ethers, peroxides are not removed.
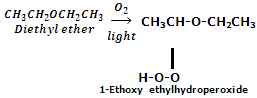
Presence of peroxides in ethers can be detected by the addition of freshly prepared FeSO4 and KSCN. Appearance of red colour indicates peroxides. Ethers can be freed from peroxides by shaking with ferrous salt solution or distilling with concentrated H2SO4.