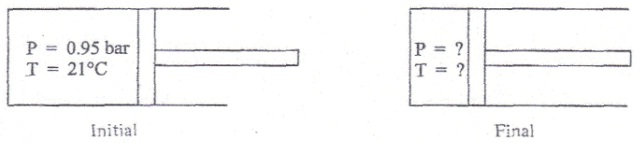
A diesel engine operates devoid of a spark plug by using the high-temperature gas produced throughout the compression stage to ignite the fuel. During a typical compression, pure air that is originally at 21 °C and 0.95 bar is reversibly and adiabatically compressed to 1/20 of its original(actual) volume by the piston, as shown in the figure below.
a) If air is assumed to obey the ideal gas equation of state, find out the pressure and temperature in the cylinder at the end of the compression. The constant-pressure heat capacity of air varies with temperature and is given by
Cp/R = 3.3 + 6x10-4
b) What is the work of compression in J/mol of air?
c) Is the ideal gas law a good approximation in this case? Why?