Assignment:
Q1. Write a structural formula for each of the following compounds:
a. N,N-diethylaminocyclopentane
b. ethylpropylamine
c. tetramethylammonium hydroxide
d. 1,3-diaminobutane
Q2. Write a correct name for each of the following compounds:
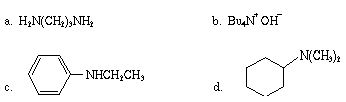
Q3. Draw the structures for and name and classify as primary, secondary, or tertiary all the isomeric amines with the molecular formula C5H13N.
Q4. Give equations for the preparation of the following amines from the indicated precursor.
a. 1-aminohexane from 1-bromopentane
b. N-butylaniline from aniline
Q5. Complete the following equations.
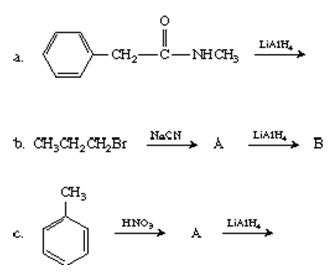
Q6. Tell which is the stronger base and why.
a. aniline or o-bromoaniline
b. aniline or cyclohexylamine
Q7. Write an equation for the reaction of
a. trimethylamine with HBr
b. cyclopentylamine with HCl
Q8. Illustrate the amphoteric nature of amino acids by writing an equation for the reaction of proline in its dipolar ion form with an equivalent of
a. hydrochloric acid, HCl
b. sodium hydroxide, NaOH
Q9. Write the formula for each of the following in its dipolar ion form.
a. leucine
b. threonine
c. methionine
d. tyrosine
Q10. Write equations for the reaction of phenylalanine with
a. CH3CH2OH and H+
b. C6H5COCl and base
c. acetic anhydride
Provide complete and step by step solution for the question and show calculations and use formulas.