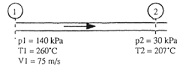
Solve the following problem:
Q: An ideal gas flows adiabatically through a duct. At section 1, p1 = 140 kPa, T1 = 260°C, and V1 = 75 m/s. Farther downstream, p2 = 30 kPa and T2 = 207°C. Calculate V2 in m/s and s2 - s1 in J/(kg · K) if the gas is
(a) Air, k = 1.4, and
(b) Argon, k = 1.67.