Questions:
1) Which atom below has 4 valence electrons?
a) Carbon
b) Beryllium
c) Hydrogen
d) Nitrogen
e) None
2) What is the correct Lewis dot structure for oxygen?
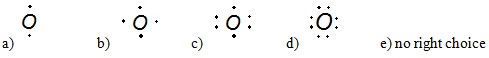
3) Which chemical has the most number of valence electrons in its Lewis structure (electron-dot structure)?
a) methane
b) hydrogen cyanide
c) dihydrogen monoxide
d) nitrogen trihydride
e) all the same
4) Which choice below has the correct Lewis structure (electron-dot structure) ?
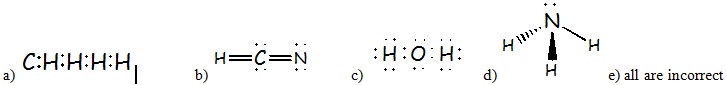
5) What is the molecular geometry of dihydrogen monoxide?
a) linear
b) trigonal planar
c) bent
d) tetrahedral
e) no right answer
6) For each of the chemical listed, place a check mark on all the type of attractive / intermolecular forces that the chemical possesses and determine if the chemical is polar or nonpolar.
|
|
Dipole-Dipole
|
LDF
|
H-Bond
|
Circle the correct choice.
|
|
methane
|
|
|
|
Nonpolar or polar
|
|
Hydrogen cyanide
|
|
|
|
Nonpolar or polar
|
|
Dihydrogen monoxide
|
|
|
|
Nonpolar or polar
|
|
Nitrogen trihydride
|
|
|
|
Nonpolar or polar
|
7. What is the molecular geometry around the carbon atom?
a) linear
b) trigonal
c) bent
d) tetrahedral
e) no right choice
8. What is the molecular geometry around the oxygen atom?
a) linear
b) trigonal
c) bent
d) tetrahedral
e) no right choice
Is the chemical polar or nonpolar?
Name all the intermolecular forces this chemical possesses.