Assignment:
1. What is the energy of a photon of violet light with a frequency of 7.35 × 1014 Hz?
2. How much greater is the energy of a photon of ultraviolet radiation (λ = 3.00 × 10-7 m) than the energy of a photon of sunlight (λ = 5.60 × 10-7 m)?
3.) (Has two parts)A light wave has a frequency of 7.57 × 1014 cycles per second.
(a) What is the wavelength?
(b) According to the table below, what color would you observe?
|
Color
|
wavelength (in 10-7 m)
|
|
Red
|
6.25 - 7.90
|
|
Orange
|
6.00 - 6.24
|
|
Yellow
|
5.77 - 5.99
|
|
Green
|
4.92 - 5.76
|
|
Blue
|
4.55 - 4.91
|
|
Violet
|
3.90 - 4.54
|
4.)At a particular location and time, sunlight is measured on a 1 m^2 solar collector with a power of 1,213.0 W. If the peak intensity of this sunlight has a wavelength of 5.58 × 10^-7 m, how many photons are arriving each second?
5.) What is the energy of a gamma photon of frequency 7.13 × 1020 Hz?
6.) At a particular location and time, sunlight is measure on a 1.00 square meter solar collector with an intensity of 1,210.0 W/m2. If the peak intensity of this sunlight has a wavelength of 5.60 × 10-7 m, how many photons are arriving each second?
7.) What is the energy of a photon of wavelength 9.73 mm?
8.) To the nearest hundredth of a minute, how many minutes are required for a radio signal to travel from the Earth to a space station on Jupiter if the planet Jupiter is 5.9 × 108 km from the Earth?
9.) To the nearest hundredth of a second, how much time is required for reflected sunlight to travel from the Moon to the Earth if the distance between the Earth and the Moon is 3.91 × 105 km?
10.) (has two parts) What is the speed of light when traveling through
(a) water = ___× 10 m/s
(b) ice? __× 10 m/s
11.) What is the frequency of light with a wavelength of 8.20 × 10-7 m?
12.) What is the energy of a photon of ultraviolet radiation with a wavelength of 1.52 × 10-7 m?
13.) Light passes through a transparent substance at a speed of 2.256 × 108 m/s.
Based on the following table, what is the material?
|
Color
|
wavelength (in 10-7 m)
|
|
Red
|
6.25 - 7.90
|
|
Orange
|
6.00 - 6.24
|
|
Yellow
|
5.77 - 5.99
|
|
Green
|
4.92 - 5.76
|
|
Blue
|
4.55 - 4.91
|
|
Violet
|
3.90 - 4.54
|
14.) At a particular location and time, sunlight is measured on a 1.00 square meter solar collector with an intensity of 1,178.0 W/m2. If the peak intensity of this sunlight has a wavelength of 5.60 × 10-7 m, how many photons are arriving each second?
15.) A neutron with mass 1.68 × 10-27 kg moves from a nuclear reactor with a velocity of 9.51 × 103 m/s. What is the deBroglie wavelength of the neutron?
16.) If the charge-to-mass ratio of a proton is 9.58 × 107 coulomb/kilogram and the charge is 1.60 × 10-19 coulomb, what is the mass of the proton?
17.) How much energy is required to completely remove the electron from a hydrogen atom in the ground state?
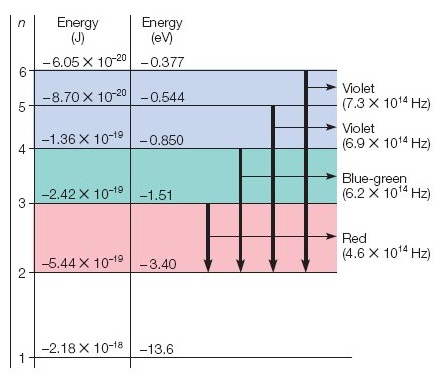
18.) (has two parts use picture below to answer this question) Referring to the figure below, how much energy is needed to move an electron in a hydrogen atom from n = 5 to n = 6? Give the answer in
(a) in joules
(b) in eV ___ eV
19.) Use chemical symbols and numbers to identify the following isotopes:
(a) Potassium-39 :
(b) Neon-22 :
(c) Tungsten-184 :
(d) Iodine-127