QUESTION 1
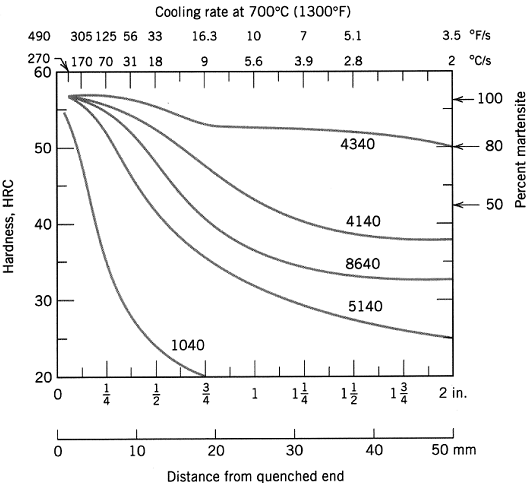
The hardenability curves for a number of steels are shown in the diagram below. Rank the steels in ascending order according to their hardenability. If the thermal properties of the steels are the same, i.e. the cooling curves (temperature versus time) at a certain distance from the quenching end are the same for all the steels, explain the difference in hardness at a distance of 30 mm from the quenched end between 5140 and 4340 by plotting schematically the C curves for each in the same continuous TTT (i.e. temperature-time-transformation) diagram and comparing the resulting microstructures.
QUESTION 2
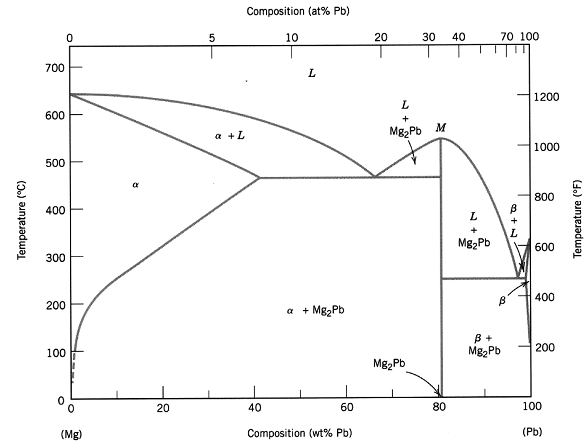
A magnesium-lead (Mg-Pb) alloy is slowly cooled (i.e. under equilibrium conditions) from 700°C. It is found by analysis that the first solid phase to solidify contains 20 wt% Pb. This alloy is further cooled to mom temperature. Using the equilibrium Mg-Pb phase diagram below, answer the following questions.
(a) What is the composition of the alloy and what is the temperature at which the first solid phase solidifies? (Estimates rounding to convenient numbers are sufficient.)
(b) What is (are) the phase(s) present at room temperature and the mass fraction of each phase.
(c) Is there any eutectic structure present at room temperature? If so, what is the mass fraction of the eutectic structure? Is there any phase present at room temperature which is not the primary phase (the first solid phase that has solidified) and does not belong to the eutectic structure? If so, what is the mass fraction of this phase.
(d) Sketch the microstructure at room temperature.
Attachment:- Materials.pdf