Discuss the below:
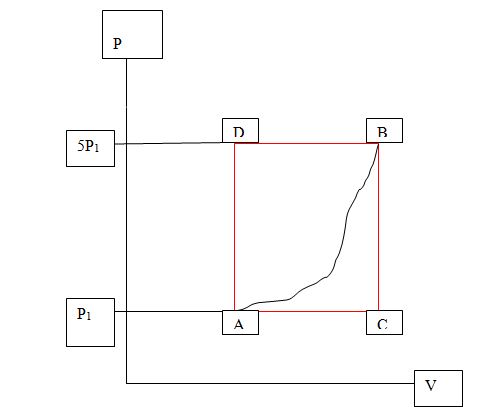
Q1) A system whose equation of state depends only on pressure P, volume V and temperature T is taken quasi-statically from state from state A to state B (in the figure shown above) along path ACB at the pressures indicated. In this process 50J of heat enter the system and 20J of work are done by the system.
a)How much heat enters the system along path ADB?
b) If the system goes from B to A along the curved path indicated on the diagram (imagine this is a smooth curve) the work done on the system is 25J how much heat enters or leaves the system?
c) If the internal energy at A is denoted by UA and the internal energy at B by UB and so on around the path Suppose that UD - UA = 25J. What then is the heat transfer involved in the process AD to DB
Q2) 10 ^-3 m^3 of lead is compressed reversibly and isothermally at room temperture from 1 to 1000 atms. Pressure.
a) How much work is done on the lead? The isothermal compressibility of lead is 2.2 x10^-6 (atm)^-1 (this is assumed independent of pressure).Use 1 atm~10^5 pa
b) What is the change in the internal energy of the lead if the amount of lead is compressed reversibly but adiabatically from 1 to 1000atm.