Question 1: Describe any five parts:
a) Platinum resistance thermometer.
b) Adiabatic lapse rate.
c) Carnot theorem.
d) Plots of heat capacity (Cp) versus temperature (T) for the first order and the second order phase transitions.
e) Sedimentation.
f) Critical constants for dieterici’s equation.
g) Sackur-Tetrode equation.
Question 2:
a) What is a thermocouple? Describe its working principle. How is it used to measure the temperature? Write down the range of temperature covered by:
i) A chromel-alumel thermocouple, and
ii) An iron-constantan thermocouple.
Describe merits and demerits of a thermocouple for temperature measurements.
b) Derive an expression of the work done by an ideal gas throughout an isothermal expansion of an ideal gas.
c) Two moles of a perfect gas occupy a volume of 0.060 m3 and exert a pressure of 4 × 105 Nm−2. It is compressed isobarically to 0.045 m3. Compute the work done by the gas.
Question 3:
a) Two kg of water is heated from 0°C to 100°C and transformed into steam reversibly. Compute the total increase in its entropy. Given, heat capacity of water = 4.2 × 103 J kg−1 K−1 and latent heat of vaporization = 2.25 × 106 J kg−1.
b) For Carnot cycle, show that the amount of heat absorbed in a reversible cycle is proportional to the temperature of source. Compute the efficiency of a Carnot engine working between temperatures 560 K and 350 K.
c) Write down the conditions for thermodynamic equilibrium of:
i) Thermally isolated – isochoric system,
ii) Thermally isolated – isobaric system,
iii) Thermally conducting – isochoric system, and
iv) Thermally conducting isobaric system.
Question 4:
a) Obtain Einstein’s formula for mean square displacement of a Brownian particle.
b) The coefficient of viscosity of helium at 300 K is 2 × 10−5 Nsm−2. Compute the diameter of a helium molecule. Take average velocity of molecules as 1.26 × 103ms−1, Avogadro number as 6 × 1023 mol−1 and atomic weight of helium as 4.
c) Illustration the assumptions made by Vander Waals for real gases. How far does the real gas equation describe the observed results?
Question 5:
a) By using the expression for rotational partition function, get an expression for heat capacity of hydrogen. Depict its temperature variation.
b) The thermodynamic probability for a Fermi-Dirac system is given by:
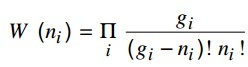
Get an expression for Fermi-Dirac distribution function. Plot the Fermi function f (ε) versus ε for a completely degenerate and a strongly degenerate system.