Assignment:
Question 1. Discuss the results of your classification tests and melting point of your derivatives. What is your unknown? How do your data exclude other possibilities and what, if anything is still inconsistent with your conclusion?
“Boiling Point for the Unknown is 218oC. For the 2,4-dinitrophenylhydrazone derivative of the unknown, the melting point is 191oC. Tollens’ test of the unknown, it shows that there are no silver mirror in the solution of ketone. For the iodoform test, it shows that it is a clear yellow liquid”
Question 2. Draw the structure of the dye formed from Primuline red and 2-naphthol. Be sure to pay close attention to regiochemistry.
Question 3. Why does indigo have to be reduced to be used as a dye?
Question 4. Draw the resonance form of N,N-dimethylaniline that is most prone to react with diazotized sulfanilic acid. Explain your answer.
The reaction is Diazotized sulfanilic acid (4-Diazobenzenesulfonic acid) + N,N-Dimethylaniline with CH3COOH/NaOH to get methyl orange
(4-[4-(Dimethylamino)phenylazo]benzenesulfonic acid, sodium salt)
Question 5. Show a step-by-step mechanism for the reaction of the diazonium ion formed from aniline with phenol. Please draw the 3-D structures of the product.
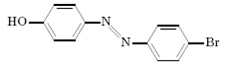
Question 6. To prepare the azo dye shown below would the better starting materials be…
a. P-aminophenol and bromobenzene
b. P-bromoaniline and phenol
Please explain your answer.