Assignment:
Projection of the fx(x2-3y2) orbital.Assume the lobes of the orbital are coplanar and the z axis is perpendicular to the page.
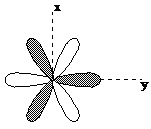
Question 1. Consider an atom having the fx(x2-3y2) orbital projection presented above. What d and p orbitals of a second atom would have the appropriate symmetry to form bonding and antibonding orbitals with this f orbital? Consider all d and p orbitals and defend your answer, including drawings. Assume the internuclear axis is the z axis and the incoming atom has the same axis system as the atom with the fx(x2-3y2) orbital projection.
Question 2. Consider an atom having the fx(x2-3y2) orbital projection presented above. What d and p orbitals of a second atom would have the appropriate symmetry to form bonding and antibonding orbitals with this f orbital? Consider all d and p orbitals and defend your answer, including drawings. Assume the internuclear axis is the y axis and the incoming atom has the same axis system as the atom with the fx(x2-3y2) orbital projection.