Assignment:
Six-coordinate CR(3) complexes of the type trans-(CrL4A2)n+, generally have magnetic moments consistent with three unpaired electrons, which suggests occupancy of the d orbitals as shown to the right. In principle, a complex with only one unpaired electron could be generated by a suitable choice of L and A such that the separation between the dxy and the dxz, dyz orbitals becomes larger than the spin pairing energy.
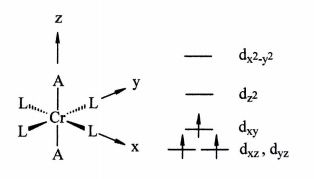
Assume that L is a sigma donor only ligand. Would strong pi donors or strong pi acceptors be the best choice for axial ligand A in order to increase the separation of the dxy and the degenerate dxz, dyz orbitals? Explain the basis for your choice being careful to:
1. Explicitly indicate the relative energies of the metal and ligand pi orbitals
2. Explicitly indicate the specific ligand orbital-metal orbital interactions that will influence the relative energies of the dxy and the degenerate dxz, dyz orbitals.
Provide complete and step by step solution for the question and show calculations and use formulas.