Discussion:
Solid State Chemistry: Lattice Energy
1) Estimate the radius of the cesium ion, Cs+. The lattice energy of cesium chloride, CsCl, is 633kJ/mol. For CsCl, the Madelung constant M is 1.763, and the Born exponent n is 10.7. The radius of Cl - is known to be 1.81 A. Express your answer in Angstroms.
2) Chlorofluorocarbons (CFC's) are organic compounds that have been implicated in ozone depletion. When the CFC known as Freon 12 (CCl2F2) is exposed to UV radiation (wavelength in the range of 10 nm to 400 nm, a bit shorter than visible light), a chlorine atom breaks off from the rest of the molecule. Prove that this is possible by calculating the maximum wavelength capable of breaking the C-Cl bond.
3) Which compound would you expect to have the highest melting point?
BeO
MgO
CaO
SrO
4) Which compound would you expect to have the lowest melting point?
MgO
BeO
NaCl
KCl
5) Identify which bond you would expect to be the most polar.
H-C
H-F
H-Cl
H-S
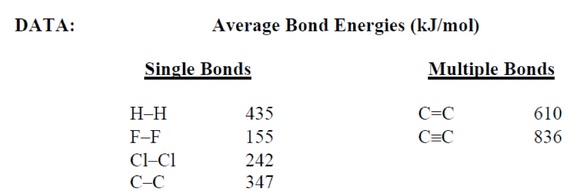
6) Answer the following questions given the bonding energy (kJ/mol) data:
1. I-I:150
2. F-F:160
3. C-C:350
4. H-H:435