Lab Experiment:
Separation of A Three Component Mixture by Extraction
Purpose: Extraction depends upon the relative solubilities of materials in different solvents. In this lab, a mixture containing a carboxylic acid, an amine and a neutral compound will be extracted in order to isolate the three compounds. Initially the mixture is dissolved in diethyl ether. 3M HCL is added, shaken and removed. In this first extraction, amines are rendered water-soluble by formation of the HCL salt. 3M NaOH is then added, shaken and removed; the carboxylic acid is rendered water-soluble by formation of the sodium salt. The neutral material remains in the ether layer.
The amine is recovered from the after phase by neutralization of the HCL salt using 10 M NaOH. The carboxylic acid is recovered from the water phase by neutralization with 12 M HCL. Finally the neutral material is recovered by evaporating the ether.
Goals:
• To isolate the components of a mixture.
• To identify the components based on melting point.
• To quantify recover base on starting mass.
• To write appropriate acid-base reactions.
• To write a flow-scheme for extraction.
Materials:
• Mixture containing an amine, a carboxylic acid, and a neutral compound
• 12 mL glass screw-cap centrifuge tube
• Diethyl ether
• 3 M HCL
• 3M NaOH
• 12M HCL
• 10M NaOH
• CaCl2 pellets
• 3- 25mL Erlenmeyer flasks
• 6 Pasteur pipettes
• Hirsch funnel with 1.5cm filter papers
• Filtration apparatus
• Gloves
• Red and blue litmus paper
• Melting point tubes and apparatus
Procedure/Observations:
A. Preparation of mixture.
1. Instructor provided a portion of the unknown mixture. The starting mixture was clear with a tint of yellow, the solid was yellowish. Vile weighed 15.5733 g, vile and unknown 15.7191 g. Unknown weighed 0.1458g
2. Tare your glass centrifuge tube and add the portion. Added approximately 5 mL diethyl ether. Tightly cap the tube and rocked the tube until the substance is dissolved.
3. Added an additional 2 mL of ether because wasn’t completely dissolved.
B. Extraction and Precipitation of the amine component.
1. To the ether mixture added approximately 2 mL 3 M HCL. Gently mixed the tube. After adding HCL the unknown was amber or pee yellow.
2. Used a clean Pastuer pipette, withdraw the bottom aqueous layer and placed it in a labeled 25 Erlenmeyer flask.
3. Repeated steps 1 and2, adding the water layer to the same flask.
4. Neutralized the acid layer by adding 10 M NaOH dropwise until the solution is alkaline to red litmus paper. Used a stirring rod to transfer a drop of the solution to the litmus paper. The component was yellow with yellow precipitate forming.
5. Placed the flask on ice.
C. Extraction and Precipitation of the carboxylic acid component.
1. To the ether mixture, add approximately 2 ml 3 M NaOH. Gently mix the tube. The bottom layer component was yellow.
2. Using a clean Pastuer pipette, withdraw the bottom aqueous layer and place it in a labeled 25 Erlenmeyer flask.
3. Repeat steps 1 and2, adding the water layer to the same flask.
4. Neutralize the acid layer by adding 12 M HCL until the solution is acid to blue litmus paper. Heat is generated, so be careful. You should see clouding of the solution as the amine precipitates out. With the addition of HCL the component color changes to barely yellow with white crystals on the top.
5. Place the flask on ice.
D. Recovery of the neutral component
1. Extracted the ether solution with two 1 mL portions of water. Discarded the water extracts into a test tube.
2. Added CaCl2 pellets until a few swirled around. Let it site for 5 –10 minutes.
3. Used a clean Pastuer pipette, transfered the ether solution to a pre-weighted 25 mL Erlenmeyer flask. Flask weighed 25.3892 g.
4. With an air stream, dried the ether in the hood. After drying the weight was 25.4488 g.
E. Recovery of the amine and carboxylic acid components.
1. Pre-weighed two watch glasses and 1.5 cm filter papers on the analytical balance. Watch glass #1 18.4998g, #2 18.7237g
2. Placed filter paper in the Hirsch funnel and wet it with some of the ice water. Turned on the vacuum.
3. Transferred the contents of the amine beaker to the Hirsch funnel and allowed the liquid to flow into the lower flask. Washed the crystals with a small portion of the ice water.
4. Removed the Hirsch funnel from the filtration flask and scrape the filter paper and crystals onto the watch glass. Set aside to dry.
5. Repeated the operation using the flask containing the carboxylic acid.
6. Disposed of the waste liquid and filter.
**When picking up the acid from the ice it was slippery and I spilled some of my solution.
Calculation of % recovery:
1. After all components are completely dry, reweighed the watch glasses and the Erlenmeyer flask.
2. Determine the amount of each component recovered and total their masses. Calculate the % recovery based on your starting mass.
Amine with watch glass weighed 18.5197g - 18.4998g = 0.0199g, substance was yellow. Carbonoxylic acid with watch glass weighed 18.7402g – 18.7237g = 0.0165g.
3. Calculate the % composition of the mixture.
Identification of the Unknown Components: Using the table provided I determined the unknown components based on their melting points.
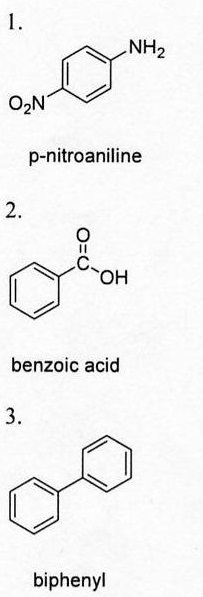
My amine melting point was 142-146, so my amine was p-nitroaniline.
My acid melting point was 118-122, so my acid was benzoic.
My neutral melting point was 67-70, so my neutral was biphenyl.
Discussion:
1. Draw the three identified structures:
2. Write the equations showing reactions of the amine and the carboxylic acid with the acids and bases added.
Reaction of amine with acid:
R-NH2 + H+ ---> R-NH3+
Reaction of carboxylic acid with base:
R-COOH + OH- ---> R-COO- + H2O
Of course, an amine won't react with a base since it is a base; and a carboxylic acid won't react with an acid since it is an acid.
3. What are the major disadvantages of using ether as a solvent?
* it's expensive
* it evaporates quickly
* it's extremely flammable (and even explosive at times)
* bad smell
* fairly poisonous (it can make you unconscious)
4. Complete the flow scheme for the extraction: