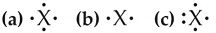Discussion:
Seeing a Lightbulb and the Attraction of an Electron to a Nucleus
We can draw an analogy between the attraction of an electron to a nucleus and seeing a lightbulb-in essence, the more nuclear charge the electron "sees," the greater the attraction.
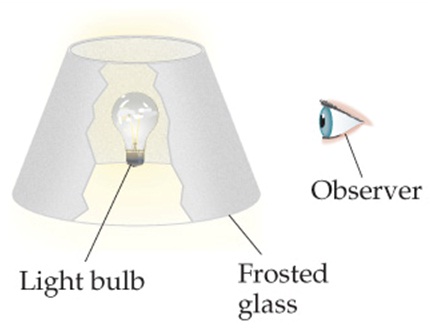
1. Within this analogy, discuss how the shielding by core electrons is analogous to putting a frosted-glass lampshade between the lightbulb and your eyes, as shown in the illustration. (See figure attached)
2. Explain how we could mimic moving to the right in a row of the periodic table by changing the wattage of the lightbulb.
3. How would you change the wattage of the bulb and/or the frosted glass to mimic the effect of moving down a column of the periodic table?
4. What is meant by the term effective nuclear charge?
5. Explain the following variations in atomic or ionic radii.
a. I- >I>I+
b. Ca2+ >Mg2+>Be2+
c. Fe >Fe2+>Be3+
6. For each of the following pairs, indicate which element has the larger first ionization energy.
a. Ti, Ba (Use electron configuration and effective nuclear charge to explain your answer)
b. Ag, Cu (Use electron configuration and effective nuclear charge to explain your answer)
c. Ge, Cl (Use electron configuration and effective nuclear charge to explain your answer)
d. Pb, Sb (Use electron configuration and effective nuclear charge to explain your answer)
7. Explain how the 4s electron in an As atom has a lower energy that 4p electron in an As atom? Also, how can we use the concept of effective nuclear charge to explain this?
8. How do we determine the factors of the order of increasing melting point?
9. For each of these Lewis symbols, (Figure attached) indicate the group in the periodic table in which the element X belongs.
Enter your answers in order given in the figure below. For example, Chlorine is in group 7A.