Assignment:
Question 1. The reversible exothermic water-gas shift reaction

, takes place in an isobaric and adiabatic PFR reactor of V=1m3. Ftot = 100 moles/sec contains 40 mol% H20 and 20 mole % of inert I. The total pressure is 3 bar and the inlet temperature is Tin = 500 k. In parallel the methanation reaction
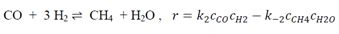
occurs. What is the CO conversion and temperature at the exit of the reactor.
Question 2. The liquid phase reaction
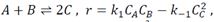
is carried out in a jacketed (cool) CSTR reactor with volume V=1m3. Initially the reactors is at a temperature of 350K and contains only species A at a concentration of 3 moles/liter (fully filled). The rate constants are at 350K are K1 (350K) =10^3 moles^-1s^-1liter and k-1 (350 K) = 10^5 moles^-1s^-1liter with activation barrier of E1=100 kJ/mol and E1=150 kJ/mol. The heat of reaction is -Kj/mol. The heat capacities for A and B are 25 J/mol/k and for C 45J/mol. a cooling medium flows around the reactor with a constant temperature of K, and UA= 100 J/s/K. The flow rate of the cooling medium is high enough that you can assume the temperature of the cooling medium to be constant. At time t=0, a constant flow of A and B at temperature of 350K is added to the reactor and the same volumetric flow rate, V=0.01m3/s of products is removed. In the moment B is added to the reactor, the reaction begins. The concentration in the incoming flow of A and B is 1mol/liter and 2 mol/liter respectively. Plot the temperature and concentration of each species as a function of time, what is the temperature and concentration of A, B, C after 1000s?
Provide complete and step by step solution for the question and show calculations and use formulas.