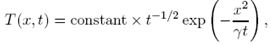Discuss the below:
Thermodynamics & Newton's law of cooling
Q: Distinguish between reversible and irreversible processes in thermodynamics. Describe the circumstances under which a) dQ = Tds and dW = -PdV.
A long cylinderical rod of radius R is attached to a source of heat at one end and its surroundings are at temperature Ro. Show that at time t, temperature of the rod T(x,t) obeys the given equation (see attachment).
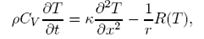
Assuming from Newton's law of cooling that R(T) = A(T - To) where A is a constant,
a) Obtain an expression for steady state temperature distribution T(x) when hot end of the bar is at temperature T1.
b) Given that A is independent of r, show that in steady state the heat transported away from the hot end is proportional to r^(3/2).
c) Given that the heat loss is negligible (A = 0), show that a solution of the equation is as given
find an expression for a constant γ