Assignment:
The equilibrium constant of the reaction A <--> 2B + C shown
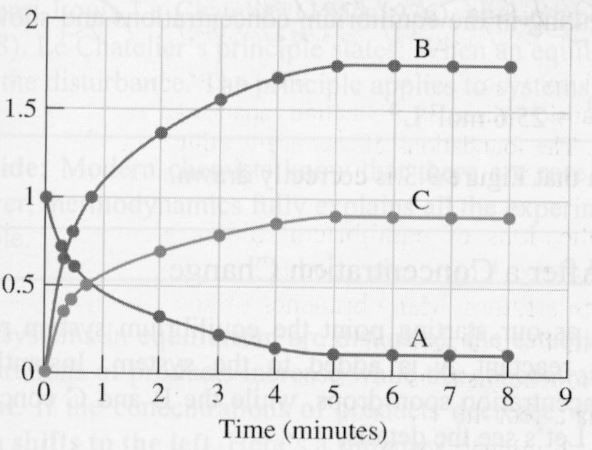
is 25.6 mol^2/L^2 at an unspecified temperature. The equilibrium concentrations in this system are [A]= 0.110 M, [B]= 1.78 M and [C]= 0.890 M. The reaction system is slugged with an additional amount of reactant, equal to a concentration of 0.270 M, instantaneously raising the [A] concentration to 0.380 M. The system then undergoes a shift and reestablishes equilibrium. Calculate the new equilibrium concentrations.
Provide complete and step by step solution for the question and show calculations and use formulas.