Assignment:
Four possible structures are shown below for the complex trans-{ML4(n^2-C2H4)2}. L is a neutral 2 electron sigma only donor. The notation 'n^2-C2H4' means that the ethylene is bonded to the metal such that both carbon atoms are interacting simultaneously with the metal atom. When M is a low-spin, d6 metal, which of the four diastereoisomers shown below is likely to be the lowest energy and why? Your answer should include an explicit description of all ligand orbital-metal orbital interactions, including the precise metal orbits that are involved.
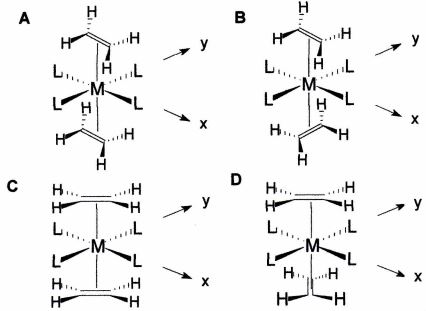
Provide complete and step by step solution for the question and show calculations and use formulas.