Assignment:
Question 1. The Equilibrium constant Kp for the reaction
2SO2(g) + O2(g) ß---à 2SO3(g)
Is 5.60 x 10^4 at 350 degrees C. The initial pressures of SO2 is 0.350 atm and the initial pressure of O2 is 0.762 atm at 350 degrees C. When a mixture equillibtrates, is the total pressure less than or greater than the sum of the initial pressures (1.112 atm)?
Question 2. You are working as a lab assistant and are tasked to prepare an acetic acid - sodium acetate buffer solution with a pH of 4.00 ± 0.02. What molar ratio of CH3COOH to CH3COONa should be used?
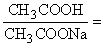
Question 3. The equilibrium constant Kc for the reaction
H2(g) + Br(g) ß-----à 2HBr(g)
Is 2.18 x 10^6 at 730 degrees C. Starting with 3.20 moles of HBr in a 12.0-L reaction vessel, calculate the concentrations of H2, Br2, and HBR at equilibrium.
Question 4. Assuming equal concentrations of conjugate base and acid, which one of the following mixtures is suitable for making a buffer solution with an optimum pH of 4.6 - 4.8?
- CH3COO2Na / CH3COOH (Ka = 1.8 x 10-5)
- NH3 / NH4Cl (Ka(NH4+) = 5.6 x 10-10)
- NaOCl / HOCl (Ka = 3.2 x 10-8)
- NaNO2 / HNO2 (Ka = 4.5 x 10-4)
- NaCl / HCl
Question 5. You have 500.0 mL of a buffer solution containing 0.20 M acetic acid (CH3COOH) and 0.30 M sodium acetate (CH3COONa). What will the pH of this solution be after the addition of 20.0 mL of 1.00 M NaOH solution?
Ka = 1.8 x 10-5
Question 6. 50.00 mL of 0.10 M HNO2 (nitrous acid) was titrated with 0.10 M KOH solution. After 25.00 mL of KOH solution was added, what was the pH in the titration flask? (Given Ka = 4.5 x 10-4)
Question 7. The solubility product for CrF3 is Ksp = 6.6 x 10-11. What is the molar solubility of CrF3?
Question 8. The Ksp for Ag3PO4 is 1.8 x 10-18. Determine the Ag+ ion concentration in a saturated solution of Ag3PO4.
Question 9. Will a precipitate of MgF2 form when 300 mL of 1.1 x 10-3 M MgCl2 solution are added to 500 mL of 1.2 x 10-3 M NaF? Ksp (MgF2) = 6.9 x 10-9