Answer THREE questions.
Question 1: Answer ALL parts of this question, which involves consideration of the drug Farxiga (1), which is used in the treatment of type 2 diabetes.
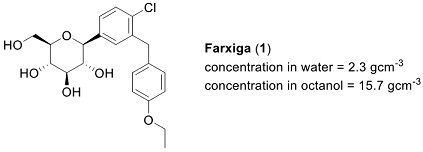
(a) Farxiga (1) was discovered, in part, using a fragment-based drug discovery approach. Briefly discuss, with the aid of a diagram, the principle of fragment-based drug discovery.
(b) The glucose sub-structure, as found in (1), may be considered by medicinal chemists as a 'privileged scaffold'. Explain what is meant by the term 'privileged scaffold' and give the structure of another example.
(c) Re-draw the structure of Farxiga (1), and clearly identify all the i) acidic, ii) basic, iii) hydrogen bond donor, iv) hydrogen bond acceptor sites in the molecule. Then suggest all the ways in which Farxiga may interact with a receptor.
(d) What is the Lipinski 'Rule of 5'? Clearly define any additional symbols or terms that you use. Does Farxiga (1) conform to the Lipinski 'Rule of 5'? [Assume C = 12; H = 1; O = 16; Cl = 35.5]
(e) What do you understand by the terms Phase 1 metabolism and Phase 2 metabolism? Use Farxiga (1) to illustrate your answer by identifying at least two possible examples of each class and drawing the structures of the appropriate metabolies.
Question 2: Answer ALL parts.
(a) In an attempt to improve the biological activity of a lead compound, which has been identified for the treatment of neurological disorders, a Hansch study was performed around compounds of general class.
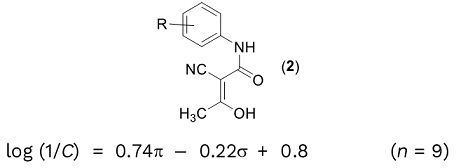
(i) Define the terms C, n, σ, and π in the Hansch equation. Comment on the reliability and accuracy of the Hansch equation. What sort of properties (in terms of σ and π) should the R group possess in order to maximize biological activity? Which property is the most important?
(ii) Explain why Hansch analysis is particularly accurate when the R group is in positions 'meta' or ',para' to the NH, but less reliable when it is in the 'ortho' position.
(iii) Discuss, in outline, the use of Craig Plots in optimising biological activity.
(iv) One concern about structures like (2) is its potential interaction with the hERG receptor. What is the hERG receptor and why is this interaction a problem? What is the pharmacophore model for general hERG interaction? Does (2) conform to this pharmacophore?
(v) Given that the pKa of a derivative of (2) is 5.5, is it more likely to be absorbed from the stomach (pH = 1.2) or intestine (pH = 6.4)?
(b) Molecular docking is widely used in hit identification and lead optimisation for drug development. Define, with examples, the concept of molecular docking.
Question 3: Answer ALL parts of this question.
(a) Explain what is meant by the terms pharmacogenomics and pharmacometabonomics.
(b) Describe how pharmacogenomics and pharmacometabonomics can help deliver personalized medicine. In particular, describe with examples the following:
(i) How genetic differences between people can affect the interaction between a drug and the patient.
(ii) How environmental factors may affect a patient's response to a drug.
Question 4: Answer ALL parts of this question.
(a) Answer BOTH parts
(i) Describe 2 different strategies for targeting the Epidermal Growth Factor signalling pathway in cancer. Include named examples of drugs developed from these strategies in your answer.
(ii) Briefly explain why screening for RAS mutations is recommended before prescribing Erbitux.
(b) cis-Platin is an example of an electrophilic cross-linking anti-cancer agent. With the aid of suitable diagrams, describe the mode of action of cis-Platin. Also explain why trans-platin is inactive.
Question 5: Answer ALL parts of this question.
(a) Briefly describe the biochemical mechanisms which are involved in and contribute to cerebral ischemic damage and stroke.
(b) A large number of clinical studies suggest that the pathophysiology of depression is associated with a dysfunction of the glutamatergic system. Discuss this statement in reference to a shift away from the monoamine theory of depression, and how pharmacological therapy of this psychiatric disorder may be changing.
Our Drug Discovery and Medicinal Chemistry Assignment Help service is for every student, who is pursuing this course and is struggling to draft a good quality paper within the deadline.
Tags: Drug Discovery and Medicinal Chemistry Assignment Help, Drug Discovery and Medicinal Chemistry Homework Help, Drug Discovery and Medicinal Chemistry Coursework, Drug Discovery and Medicinal Chemistry Solved Assignments