Assignment:
In normal phase column chromatography, which solvent has more eluting power: petroleum ether or dicloromethane? In what way is the eluting power of a solvent related to its polarity? In normal phase column chromatography, how does the principle of like dissolve like explain the affinity of a compound for the mobile phase, relative to the stationary phase.
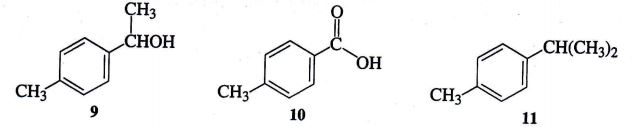
A mixture containing compounds 9,10,11 was separated by normal phase column chromatography, using neutral alumina as the stationary phase, and diethyl ether as the mobile phase. Label each compound according to its relative polarity (nonpolar, slightly polar, moderately polar, and/or very polar). Predict the order (1st,2nd, 3rd) in which the compounds 9,10 and 11 will elute from the column. Diagrams of these compounds.
Provide complete and step by step solution for the question and show calculations and use formulas.