Assignment:
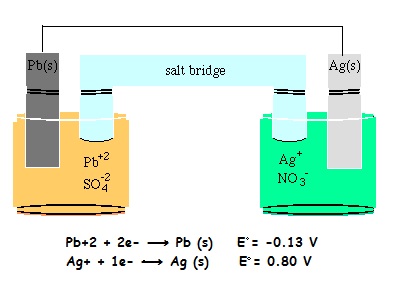
Pb(s)| Pb2+ (1M) || Ag+ (1M) | Ag(s)
Determine which of the following statements about the cell shown are True or False.
- The silver half-cell is the anode.
- The mass of the silver electrode is increasing.
- Electrons are spontaneously produced in the lead half-cell.
- As the reaction proceeds, the concentration of the silver ions decreases.
- The standard cell potential, ξo, equals 1.73 V.
- Cations move from the lead half-cell to the silver half-cell.
- The lead electrode is the anode.
- The cell, as represented by the line notation, is an electrolytic cell.
Provide complete and step by step solution for the question and show calculations and use formulas.