Assignment:
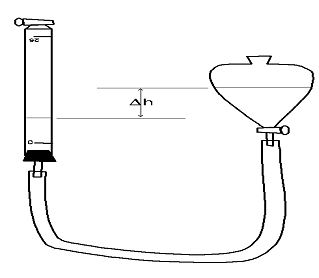
Lab performed Boyle’s Law:
Information:
Boyle’s Law states that for a constant temperature,
P1V 1=P2V 2
Which can be rearranged to give PV =k where k is some constant value.
Compare this to the Ideal Gas Law, PV =n RT
This was the used Procedure:
Holding the separator funnel roughly level with the buret, pour water into it from a beaker. The water should travel through the plastic tubing and rise into the buret. Stop when the water reaches a level between the 25mL and 20 mL marks. (A larger initial volume will provide a larger change in volume for a given change in pressure.) Raise or lower the separator funnel until the level in the buret is again between the 20 and 25 mL marks and close the petcock on the buret. This should seal a specific amount of air into the system.
Data Collection:
The difference in water levels between the separatory funnel (marked Δh on the diagram) and the buret serves to change the pressure of the trapped air, and is used to measure it, the same as a U-manometer. The total air pressure in the buret is found using the formula Ptotal =Patm +ρwater g Δh
Notice that Δh is positive if the level in the separator funnel is higher than the level in the buret, and negative if it is lower.
Total volume is a little more complicated in concept: we have to count the volume between the 25 mL mark and the bottom of the buret, and we need to correct for the fact that the buret markings are “backward”, namely that the maximum volume exists at the
0 mark and the minimum exists at the 25 mL mark.
The formula to use is Vtotal=V ds+25.00 mL−Vburet
Where Vds is the “dead space” volume next to the buret’s petcock and Vburet is the buret reading. (Use 2.0 mL as the dead space volume if a specific measurement is not available, Don’t forget to change the volumes to m3! There are 106 mL in one cubic meter.
Data are collected by changing the level of the separator funnel relative to the buret and then recording the volume and Δh each time. Be sure to read the ruler and the buret as accurately as possible.
Data Processing:
Once the figures for volume and absolute pressure are found, just multiply them together for each data point. Remember that PV should be constant! (If it’s not, the two most likely problems are air leaks or using the wrong volume for the dead space) Find the average and standard deviation for PV.
Next, you can calculate the number of moles of gas trapped in the buret. Using your average value for PV, calculate n= PV/RT.
Please review the document enclosed here:
Provide complete and step by step solution for the question and show calculations and use formulas.
Attachment:- Graphs for Physics Lab-Boyles Law.rar