Assignment:
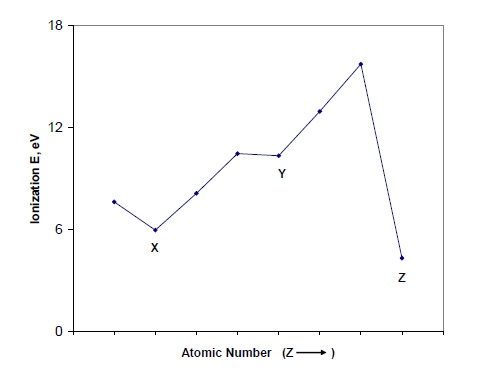
Question 1. The Figure below shows the first ionization of a sequence of eight atoms from the Periodic Table, but not necessarily from the same row. The other given is that N, O and F occur within the run. Identify all elements involved. Explain your reasoning, and explain why the ionization energies of atoms X, Y and Z all appear as local minima in the overall sawtooth pattern.
Question 2. Why does the first ionization energy increase in the following order?
Al< Ga < B
Question 3. The entropy of hydration becomes more negative from Na+ to Mg2+ to Al3+ though the coordination number is probably 6 in each case. Account for the trend in disorder.
S? 298 (J mol-1 K-1) : Na+(aq), 60.3; Mg2+(aq), -138; Al3+(aq), -322.