Assignment:
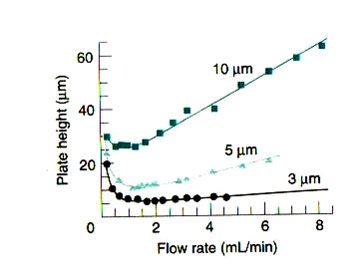
Question 1. Based on the following figure explain which particle size produce the best resolution in HPLC and why.
Question 2. To find the Ce4+ content in a solid, 4.37 g were dissolved and treated with and excess of iodate to precipitate Ce(IO3)4. The precipitate was filtered, washed and dried and ignited in air to convert the cerium iodate to ceria (CeO2). If 0.104 grams of ceria were produced, what is the weight percent of cerium in the solid?
(MW: Ce - 140.115, I - 126.904, O - 15.999)
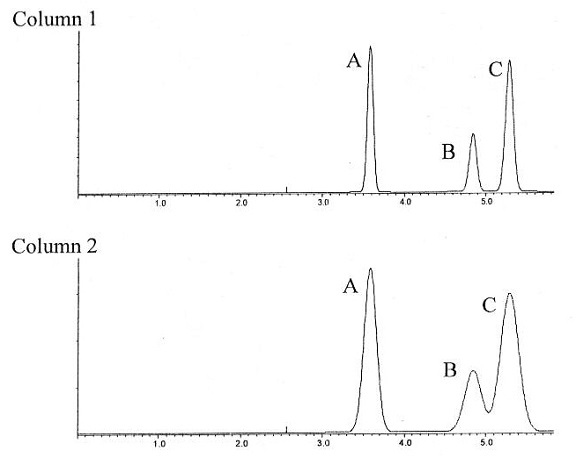
Question 3. Use the chromatographs below to answer the following questions (Circle your answer). The two chromatograms of samples containing A, B, and C were obtained at the same flow rate with two columns of equal length.
a) Which column has more theoretical plates? Column 1 or Column 2
b) Which compound has the smallest partition coefficient? Sample A, Sample B, or Sample C
c) Which column gives better resolution? Column 1 or Column 2
d) If these were HPLC columns, which column has smaller particles? Column 1 or Column 2
Question 4. A 2.50 mL aliquot of a solution that contains 3.8 ppm iron (III) is treated with an appropriate excess of KSCN and diluted to 50.0 mL. What is the absorbance of the resulting solution at 580 nm in a 2.50 cm cell? The complex FeSCN2+ has a molar absorptivity of 7.00×103 L cm-1mol-1. (Fe - 55.845 g/mol).
Provide complete and step by step solution for the question and show calculations and use formulas.