Discuss the below:
Q1 A Ping-Pong ball has a diameter of 3.8 cm and average density of 0.084 g/cm3. What force is required to hold it completely submerged under water?
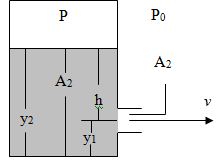
Q2. A tank with a cover, containing a liquid of density p, has a hole in its side at the distance y1 from the bottom. The diameter of the hole is small relatively to the diameter of the tank. The air above the liquid is maintained at a pressure P. Assume steady frictionless flow and show that the speed at which the fluid leaves the hole when the liquid is a distance h above the whole is
v = √ [2(P-P0) +2gh]/p
Q3. In a time t, N hailstones strike a glass window of area A at angle θ to the window surface. Each hailstone has a mass m and a speed v. If the collisions are elastic, what are the average force and the pressure on the window?
Q4. Use the definition of Avogadro's number to find the mass of a helium atom.
Q5. A 5 L vessel contains nitrogen gas at 27ºC and 3 atm. Find the total kinetic energy of gas molecules and the average kinetic energy per molecule.
Q6. Suppose you breathe out 22 breaths per minute, each with volume of 0.6 L. Assume you inhale dry air and exhale air at 37ºC containing water vapor with a vapor pressure of 3.2kPa. The vapor comes from evaporation of liquid water in your body. Model the water vapor as an ideal gas. Assume its latent heat of evaporation at 37ºC is the same as its heat of evaporation at 100ºC (λ = 2.26*106 j/kg). Calculate the rate of your energy loss by exhaling humid air.
Q7. 5 mol of air at 20ºC and 1 atm are compressed to one tenth of the original volume by (a) an adiabatic process (Pvgamma = const, where γ = Cp/Cv) and (b) an isothermal process (PV=const). What is the final pressure and temperature in each of these cases?
Q8. A Carnot engine has a useful power output of 150 kW. The engine operates between two reservoirs at 20ºC and 500ºC. How much energy does it absorb per hour? How much energy is expelled per hour in its exhaust?
Q9. If the mass mh of water at Th is poured into an aluminum cup of mass mAl containing mass mc of water at Tc, where Th>Tc, what is the equilibrium temperature system?