Assignment:
Q1. Write a structural formula for each of the following compounds:
a. isopropyl methyl ether
b. trans-2-pentene oxide
c. 3-ethoxyhexane
d. ethylene glycol dipropyl ether
Q2. Name each of the following compounds.
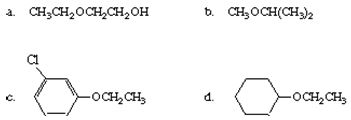
Q3. Ethers and alcohols can be isomeric. Write the structures, and give the names for all possible isomers with the molecular formula C4H10O.
Q4. Write equations for the reaction of each of the following with (1) Mg in ether followed by (2) addition of D2O to the resulting solution.
a. (CH3)2CH CH2Br
b. CH3CH2OCH2CBr(CH3)2
Q5. Write equations for the best method to prepare each of the following ethers:
a. CH3CH2CH2OCH2CH3
b. (CH3)2CHCH2OCH2CH(CH3)2
Q6. Write an equation for each of the following reactions. If no reaction occurs, say so.
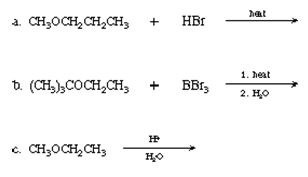
Q7. Write equations to show how 1-pentanol can be synthesized from bromopropane and ethylene oxide, using a Grignard reagent.
Provide complete and step by step solution for the question and show calculations and use formulas.