Assignment:
A certain experimental procedure is as follows:
1. You react an unknown alcohol with acetic anhydride in a condenser. (The temperature of the alcohol solution drops with the addition of acetic anhydride).
2. Sulfuric acid is then added with the alcohol and the acetic anhydride in the condenser. (This causes an exothermic reaction).
3. After the temperature of the above reaction has dropped back down to room temperature, the mixture is transferred to a separatory funnel, using a 50 mL diethyl ether rinse to aid the transfer
4. The ether solution is washed with ice cold 5% NaOH.
5. After the last extraction, the pH of the organic solution is checked to be sure it is basic. The organic portion is then washed with saturated sodium chloride solution, dried with sodium sulfate, and filtered into a 100 mL roundbottom flask.
6. The ether and the product are fractionally distilled.
It turns out that product that is fractionally distilled (along with the ether) in the end is isoamyl acetate (also called isopentyl acetate). This implies that the reaction between the unknown alcohol and the acetic anhydride produced isoamyl acetate.
Questions:
Q1. This shows stepwise how an ester is formed from the reaction of an alcohol and acetic anhydride. Substitute the alkyl group you have determined for R, and draw in curved arrows to show the movement of electrons in the reactions.
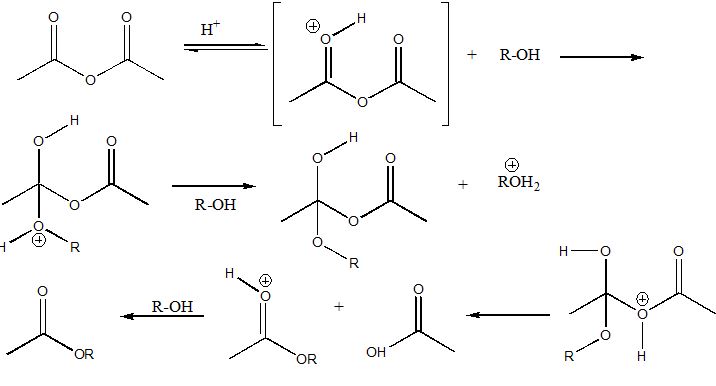
Q2.What is the structure of your unknown alcohol, and how did you arrive at this conclusion?
Q3.How would not adding sulfuric acid to the reaction mixture affect your results?
Q4. What is the theoretical % yield of your ester?
Provide complete and step by step solution for the question and show calculations and use formulas.