Assignment:
Question 1. Use the values of ?Hof given below to calculate (in kJ) ?Horxn for the following reaction:
C2H5OH (l)+ 3O2(g) → 2CO2(g) + 3H2O(g)
|
Given:
|
?Hof (kJ/mol)
|
|
C2H5OH (l)
|
-278
|
|
O2(g)
|
0
|
|
CO2(g)
|
-394
|
|
H2O(g)
|
-242
|
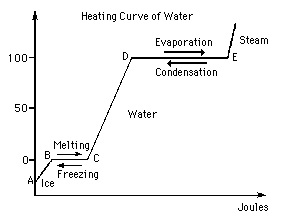
Question 2. The graph below shows the heating curve of water. Use this graph to answer the following question.
How much heat (q) is required to heat 16 g of ice at -13.0oC (Celsius) to 16 g of water at 65.0oC?
spec. heat of H2O = 4.184 J/goC
spec. heat of ice = 2.087 J/goC
Heat of Fusion = 6.02 kJ/mol
1 mol of water = 18.0g