Assignment:
Question 1. The graph at right shows the temperature of 350 grams of an unknown substance as it is cooled from a gas to a solid by extracting a constant amount of energy per minute. Assume that all the changes on the graph occur at round numbers.
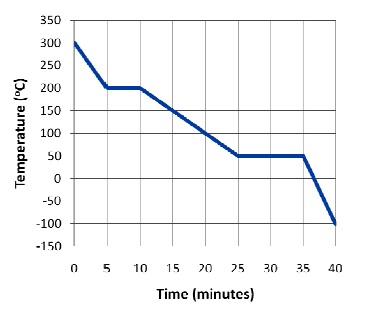
a. Which phase of the substance has the highest specific heat? Explain your answer.
b. Which is higher for this substance, the latent heat of fusion or the latent heat of vaporization? Explain your answer.
c. For this substance, cliquid is found to be 1.20 J/goC. What is the power given off by this substance, in watts?
d. Using the graph and your answer from part c, find csolid and Lf.
e. How much heat would be needed to heat 14.5 kg of this substance from –200oC to 100oC, in joules?